Intra- and inter-islet synchronization of metabolically driven insulin secretion
- PMID: 15834002
- PMCID: PMC1366509
- DOI: 10.1529/biophysj.104.055681
Intra- and inter-islet synchronization of metabolically driven insulin secretion
Abstract
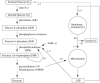
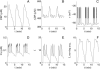
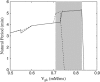
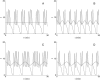
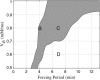
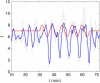
Insulin secretion from pancreatic beta-cells is pulsatile with a period of 5-10 min and is believed to be responsible for plasma insulin oscillations with similar frequency. To observe an overall oscillatory insulin profile it is necessary that the insulin secretion from individual beta-cells is synchronized within islets, and that the population of islets is also synchronized. We have recently developed a model in which pulsatile insulin secretion is produced as a result of calcium-driven electrical oscillations in combination with oscillations in glycolysis. We use this model to investigate possible mechanisms for intra-islet and inter-islet synchronization. We show that electrical coupling is sufficient to synchronize both electrical bursting activity and metabolic oscillations. We also demonstrate that islets can synchronize by mutually entraining each other by their effects on a simple model "liver," which responds to the level of insulin secretion by adjusting the blood glucose concentration in an appropriate way. Since all islets are exposed to the blood, the distributed islet-liver system can synchronize the individual islet insulin oscillations. Thus, we demonstrate how intra-islet and inter-islet synchronization of insulin oscillations may be achieved.
Figures
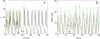
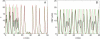
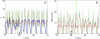

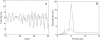
References
-
- Chou, H. F., and E. Ipp. 1990. Pulsatile insulin secretion in isolated Rat islets. Diabetes. 39:112–117. - PubMed
-
- Sturis, J., W. L. Pugh, J. Tang, D. M. Ostrega, J. S. Polonsky, and K. S. Polonsky. 1994. Alterations in pulsatile insulin secretion in the Zucker diabetic fatty rat. Am. J. Physiol. Endocrinol. Metab. 267:E250–E259. - PubMed
-
- Pørksen, N. 2002. The in vivo regulation of pulsatile insulin secretion. Diabetologia. 45:3–20. - PubMed
-
- Longo, E. A., K. Tornheim, J. T. Deeney, B. A. Varnum, D. Tillotson, M. Prentki, and B. E. Corkey. 1991. Oscillations in cytosolic free Ca2+, oxygen consumption, and insulin secretion in glucose-stimulated Rat pancreatic islets. J. Biol. Chem. 266:9314–9319. - PubMed
-
- Tornheim, K. 1997. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes. 46:1375–1380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

