Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria
- PMID: 15837212
- PMCID: PMC10994215
- DOI: 10.1016/j.vaccine.2004.12.019
Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria
Abstract
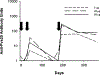
Plasmodium vivax is responsible for the majority of malaria cases outside of Africa, and results in substantial morbidity. Transmission blocking vaccines are a potentially powerful component of a multi-faceted public health approach to controlling or eliminating malaria. We report the first phase 1 clinical trial of a P. vivax transmission blocking vaccine in humans. The Pvs25H vaccine is a recombinant protein derived from the Pvs25 surface antigen of P. vivax ookinetes. The protein was expressed in Saccharomyces cerevisiae, purified, and adsorbed onto Alhydrogel. Ten volunteers in each of three dose groups (5, 20, or 80 microg) were vaccinated by intramuscular injection in an open-label study at 0, 28 and 180 days. No vaccine-related serious adverse events were observed. The majority of adverse events causally related to vaccination were mild or moderate in severity. Injection site tenderness was the most commonly observed adverse event. Anti-Pvs25H antibody levels measured by ELISA peaked after the third vaccination. Vaccine-induced antibody is functionally active as evidenced by significant transmission blocking activity in the membrane feeding assay. Correlation between antibody concentration and degree of inhibition was observed. Pvs25H generates transmission blocking immunity in humans against P. vivax demonstrating the potential of this antigen as a component of a transmission blocking vaccine.
Figures


References
-
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 2001;64(Suppl. 1–2):97–106. - PubMed
-
- Richie TL, Saul A. Progress and challenges for malaria vaccines. Nature 2002;415(6872):694–701. - PubMed
-
- Stowers A, Carter R. Current developments in malaria transmission-blocking vaccines. Expert Opin Biol Ther 2001;1(4):619–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

