Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis
- PMID: 15837802
- PMCID: PMC2171871
- DOI: 10.1083/jcb.200501156
Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis
Abstract
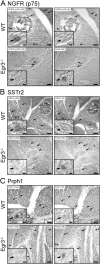
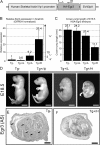
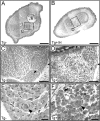
Vertebrate muscle spindle stretch receptors are important for limb position sensation (proprioception) and stretch reflexes. The structurally complex stretch receptor arises from a single myotube, which is transformed into multiple intrafusal muscle fibers by sensory axon-dependent signal transduction that alters gene expression in the contacted myotubes. The sensory-derived signal transduction pathways that specify the fate of myotubes are very poorly understood. The zinc finger transcription factor, early growth response gene 3 (Egr3), is selectively expressed in sensory axon-contacted myotubes, and it is required for normal intrafusal muscle fiber differentiation and spindle development. Here, we show that overexpression of Egr3 in primary myotubes in vitro leads to the expression of a particular repertoire of genes, some of which we demonstrate are also regulated by Egr3 in developing intrafusal muscle fibers within spindles. Thus, our results identify a network of genes that are regulated by Egr3 and are involved in intrafusal muscle fiber differentiation. Moreover, we show that Egr3 mediates myotube fate specification that is induced by sensory innervation because skeletal myotubes that express Egr3 independent of other sensory axon regulation are transformed into muscle fibers with structural and molecular similarities to intrafusal muscle fibers. Hence, Egr3 is a target gene that is regulated by sensory innervation and that mediates gene expression involved in myotube fate specification and intrafusal muscle fiber morphogenesis.
Figures



References
-
- Arber, S., D.R. Ladle, J.H. Lin, E. Frank, and T.M. Jessell. 2000. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 101:485–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

