Structural and kinetic basis for heightened immunogenicity of T cell vaccines
- PMID: 15837811
- PMCID: PMC2213140
- DOI: 10.1084/jem.20042323
Structural and kinetic basis for heightened immunogenicity of T cell vaccines
Abstract
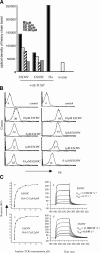
Analogue peptides with enhanced binding affinity to major histocompatibility class (MHC) I molecules are currently being used in cancer patients to elicit stronger T cell responses. However, it remains unclear as to how alterations of anchor residues may affect T cell receptor (TCR) recognition. We correlate functional, thermodynamic, and structural parameters of TCR-peptide-MHC binding and demonstrate the effect of anchor residue modifications of the human histocompatibility leukocyte antigens (HLA)-A2 tumor epitope NY-ESO-1(157-165)-SLLMWITQC on TCR recognition. The crystal structure of the wild-type peptide complexed with a specific TCR shows that TCR binding centers on two prominent, sequential, peptide sidechains, methionine-tryptophan. Cysteine-to-valine substitution at peptide position 9, while optimizing peptide binding to the MHC, repositions the peptide main chain and generates subtly enhanced interactions between the analogue peptide and the TCR. Binding analyses confirm tighter binding of the analogue peptide to HLA-A2 and improved soluble TCR binding. Recognition of analogue peptide stimulates faster polarization of lytic granules to the immunological synapse, reduces dependence on CD8 binding, and induces greater numbers of cross-reactive cytotoxic T lymphocyte to SLLMWITQC. These results provide important insights into heightened immunogenicity of analogue peptides and highlight the importance of incorporating structural data into the process of rational optimization of superagonist peptides for clinical trials.
Figures



Similar articles
-
In silico and cell-based analyses reveal strong divergence between prediction and observation of T-cell-recognized tumor antigen T-cell epitopes.J Biol Chem. 2017 Jul 14;292(28):11840-11849. doi: 10.1074/jbc.M117.789511. Epub 2017 May 23. J Biol Chem. 2017. PMID: 28536262 Free PMC article.
-
Altered peptide ligands revisited: vaccine design through chemically modified HLA-A2-restricted T cell epitopes.J Immunol. 2014 Nov 15;193(10):4803-13. doi: 10.4049/jimmunol.1400800. Epub 2014 Oct 13. J Immunol. 2014. PMID: 25311806 Free PMC article.
-
Structural basis for ineffective T-cell responses to MHC anchor residue-improved "heteroclitic" peptides.Eur J Immunol. 2015 Feb;45(2):584-91. doi: 10.1002/eji.201445114. Epub 2014 Dec 28. Eur J Immunol. 2015. PMID: 25471691 Free PMC article.
-
MM-GBSA binding free energy decomposition and T cell receptor engineering.J Mol Recognit. 2010 Mar-Apr;23(2):142-52. doi: 10.1002/jmr.1005. J Mol Recognit. 2010. PMID: 20151417 Review.
-
Structural and dynamic control of T-cell receptor specificity, cross-reactivity, and binding mechanism.Immunol Rev. 2012 Nov;250(1):10-31. doi: 10.1111/j.1600-065X.2012.01165.x. Immunol Rev. 2012. PMID: 23046120 Review.
Cited by
-
Generation and characterization of HLA-A2 transgenic mice expressing the human TCR 1G4 specific for the HLA-A2 restricted NY-ESO-1157-165 tumor-specific peptide.J Immunother Cancer. 2021 Jun;9(6):e002544. doi: 10.1136/jitc-2021-002544. J Immunother Cancer. 2021. PMID: 34088742 Free PMC article.
-
Monitoring the Dynamics of T Cell Clonal Diversity Using Recombinant Peptide:MHC Technology.Front Immunol. 2013 Jul 3;4:170. doi: 10.3389/fimmu.2013.00170. eCollection 2013. Front Immunol. 2013. PMID: 23840195 Free PMC article.
-
Tumor rejection properties of gp100209-specific T cells correlate with T cell receptor binding affinity towards the wild type rather than anchor-modified antigen.Mol Immunol. 2021 Jul;135:365-372. doi: 10.1016/j.molimm.2021.05.001. Epub 2021 May 11. Mol Immunol. 2021. PMID: 33990005 Free PMC article.
-
Dendritic cell-based vaccines: barriers and opportunities.Future Oncol. 2012 Oct;8(10):1273-99. doi: 10.2217/fon.12.125. Future Oncol. 2012. PMID: 23130928 Free PMC article. Review.
-
SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens.Virology. 2006 Mar 30;347(1):127-39. doi: 10.1016/j.virol.2005.11.042. Epub 2006 Jan 4. Virology. 2006. PMID: 16387339 Free PMC article.
References
-
- Rosenberg, S.A., J.C. Yang, D.J. Schwartzentruber, P. Hwu, F.M. Marincola, S.L. Topalian, N.P. Restifo, M.E. Dudley, S.L. Schwarz, P.J. Spiess, et al. 1998. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 4:321–327. - PMC - PubMed
-
- Apostolopoulos, V., M. Yu, I.F. McKenzie, and I.A. Wilson. 2000. Structural implications for the design of molecular vaccines. Curr. Opin. Mol. Ther. 2:29–36. - PubMed
-
- Apostolopoulos, V., M. Yu, A. Corper, L. Teyton, G. Pietersz, I. McKenzie, I. Wilson, and M. Plebanski. 2002. Crystal structure of a non-canonical low-affinity peptide complexed with MHC class I: a new approach for vaccine design. J. Mol. Biol. 318:1293–1305. - PubMed
-
- Madden, D.R. 1995. The three-dimensional structure of peptide-MHC complexes. Annu. Rev. Immunol. 13:587–622. - PubMed
-
- Rudolph, M.G., J.G. Luz, and I.A. Wilson. 2002. Structural and thermodynamic correlates of T cell signalling. Annu. Rev. Biophys. Biomol. Struct. 31:121–149. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

