Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues
- PMID: 15837816
- PMCID: PMC2213150
- DOI: 10.1084/jem.20042558
Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues
Abstract
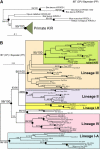
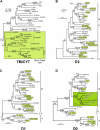
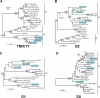
Expression of killer cell Ig-like receptors (KIRs) diversifies human natural killer cell populations and T cell subpopulations. Whereas the major histocompatibility complex class I binding functions of inhibitory KIR are known, specificities for the activating receptors have resisted analysis. To understand better activating KIR and their relationship to inhibitory KIR, we took the approach of reconstructing their natural history and that of Ly49, the analogous system in rodents. A general principle is that inhibitory receptors are ancestral, the activating receptors having evolved from them by mutation. This evolutionary process of functional switch occurs independently in different species to yield activating KIR and Ly49 genes with similar signaling domains. Selecting such convergent evolution were the signaling adaptors, which are older and more conserved than any KIR or Ly49. After functional shift, further activating receptors form through recombination and gene duplication. Activating receptors are short lived and evolved recurrently, showing they are subject to conflicting selections, consistent with activating KIR's association with resistance to infection, reproductive success, and susceptibility to autoimmunity. Our analysis suggests a two-stage model in which activating KIR or Ly49 are initially subject to positive selection that rapidly increases their frequency, followed by negative selection that decreases their frequency and leads eventually to loss.
Figures




References
-
- Biron, C.A., K.B. Nguyen, G.C. Pien, L.P. Cousens, and T.P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189–220. - PubMed
-
- French, A.R., and W.M. Yokoyama. 2003. Natural killer cells and viral infections. Curr. Opin. Immunol. 15:45–51. - PubMed
-
- Diefenbach, A., and D.H. Raulet. 2002. The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol. Rev. 188:9–21. - PubMed
-
- Dupont, B., and K.C. Hsu. 2004. Inhibitory killer Ig-like receptor genes and human leukocyte antigen class I ligands in haematopoietic stem cell transplantation. Curr. Opin. Immunol. 16:634–643. - PubMed
-
- Moffett-King, A. 2002. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2:656–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

