Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory
- PMID: 15838708
- PMCID: PMC11042470
- DOI: 10.1007/s00262-004-0602-0
Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory
Abstract
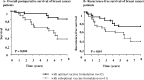
For active specific immunotherapy of cancer patients, we designed the autologous virus-modified tumor cell vaccine ATV-NDV. The rationale of this vaccine is to link multiple tumor-associated antigens (TAAs) from individual patient-derived tumor cells with multiple danger signals (DS) derived from virus infection (dsRNA, HN, IFN-alpha). This allows activation of multiple innate immune responses (monocytes, dendritic cells, and NK cells) as well as adaptive immune responses (CD4 and CD8 memory T cells). Preexisting antitumor memory T cells from cancer patients could be activated by antitumor vaccination with ATV-NDV as seen by augmentation of antitumor memory delayed-type hypersensitivity (DTH) responses. In a variety of phase II vaccination studies, an optimal formulation of this vaccine could improve long-term survival beyond what is seen in conventional standard therapies. A new concept is presented which proposes that a certain threshold of antitumor immune memory plays an important role (1) in the control of residual tumor cells which remain after most therapies and (2) for long-term survival of treated cancer patients. This immune memory is T-cell based and most likely maintained by persisting TAAs from residual dormant tumor cells. Such immune memory was prominent in the bone marrow in animal tumor models as well as in cancer patients. Immunization with a tumor vaccine in which individual TAAs are combined with DS from virus infection appears to have a positive effect on antitumor immune memory and on patient survival.
Figures

Similar articles
-
Autologous tumor vaccine modified with recombinant new castle disease virus expressing IL-7 promotes antitumor immune response.J Immunol. 2014 Jul 15;193(2):735-45. doi: 10.4049/jimmunol.1400004. Epub 2014 Jun 18. J Immunol. 2014. PMID: 24943214
-
Tumor antigen-dependent and tumor antigen-independent activation of antitumor activity in T cells by a bispecific antibody-modified tumor vaccine.Clin Dev Immunol. 2010;2010:423781. doi: 10.1155/2010/423781. Epub 2011 Mar 1. Clin Dev Immunol. 2010. PMID: 21403859 Free PMC article. Review.
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
-
Evaluation of T-Cell Responses Against Shared Melanoma Associated Antigens and Predicted Neoantigens in Cutaneous Melanoma Patients Treated With the CSF-470 Allogeneic Cell Vaccine Plus BCG and GM-CSF.Front Immunol. 2020 Jun 5;11:1147. doi: 10.3389/fimmu.2020.01147. eCollection 2020. Front Immunol. 2020. PMID: 32582212 Free PMC article. Clinical Trial.
-
Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells.Cancer Res. 2004 Nov 1;64(21):8057-61. doi: 10.1158/0008-5472.CAN-04-1545. Cancer Res. 2004. PMID: 15520216
Cited by
-
Longitudinal analysis of DC subsets in patients with ovarian cancer: Implications for immunotherapy.Front Immunol. 2023 Feb 10;14:1119371. doi: 10.3389/fimmu.2023.1119371. eCollection 2023. Front Immunol. 2023. PMID: 36845155 Free PMC article.
-
Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells.Immunology. 2005 Jul;115(3):325-36. doi: 10.1111/j.1365-2567.2005.02163.x. Immunology. 2005. PMID: 15946250 Free PMC article.
-
Cancer vaccines: on the threshold of success.Expert Opin Emerg Drugs. 2008 Jun;13(2):295-308. doi: 10.1517/14728214.13.2.295. Expert Opin Emerg Drugs. 2008. PMID: 18537522 Free PMC article. Review.
-
Cancer Vaccines in the Immunotherapy Era: Promise and Potential.Vaccines (Basel). 2023 Nov 29;11(12):1783. doi: 10.3390/vaccines11121783. Vaccines (Basel). 2023. PMID: 38140187 Free PMC article. Review.
-
Functionalized carbon nanotubes for potential medicinal applications.Drug Discov Today. 2010 Jun;15(11-12):428-35. doi: 10.1016/j.drudis.2010.04.005. Epub 2010 May 6. Drug Discov Today. 2010. PMID: 20451656 Free PMC article. Review.
References
-
- Heicappell R, Schirrmacher V, von Hoegen P, et al. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I. Parameters for optimal therapeutic effects. Int J Cancer. 1986;37:569. - PubMed
-
- Plaksin D, Progador A, Vadai E, et al. Effective anti metastatic melanoma vaccination with tumor cells transfected with MHC genes and/or infected with Newcastle Disease Virus (NDV) Int J Cancer. 1994;59:796. - PubMed
-
- Shoham J, Hirsch R, Zakay-Rones Z, et al. Augmentation of tumor cell immunogenicity by viruses—an approach to specific immunotherapy of cancer. Nat Immunol Cell Growth Regul. 1990;9:165. - PubMed
-
- Bier H, Armonat G, Bier J, et al. Postoperative active-specific immunotherapy of lymph node micrometastasis in a Guinea pig tumor model. Otorhinopharyngology. 1989;51:197. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

