Differentiation of insulin-producing cells from human neural progenitor cells
- PMID: 15839736
- PMCID: PMC1087208
- DOI: 10.1371/journal.pmed.0020103
Differentiation of insulin-producing cells from human neural progenitor cells
Abstract
Background: Success in islet-transplantation-based therapies for type 1 diabetes, coupled with a worldwide shortage of transplant-ready islets, has motivated efforts to develop renewable sources of islet-replacement tissue. Islets and neurons share features, including common developmental programs, and in some species brain neurons are the principal source of systemic insulin.
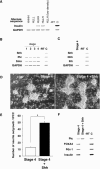
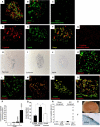
Methods and findings: Here we show that brain-derived human neural progenitor cells, exposed to a series of signals that regulate in vivo pancreatic islet development, form clusters of glucose-responsive insulin-producing cells (IPCs). During in vitro differentiation of neural progenitor cells with this novel method, genes encoding essential known in vivo regulators of pancreatic islet development were expressed. Following transplantation into immunocompromised mice, IPCs released insulin C-peptide upon glucose challenge, remained differentiated, and did not form detectable tumors.
Conclusion: Production of IPCs solely through extracellular factor modulation in the absence of genetic manipulations may promote strategies to derive transplantable islet-replacement tissues from human neural progenitor cells and other types of multipotent human stem cells.
Conflict of interest statement
Figures



References
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;242:230–238. - PubMed
-
- Edlund H. Pancreatic organogenesis—Developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. - PubMed
-
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science. 2002;296:1118–1120. - PubMed
-
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila . Curr Biol. 2002;12:1293–1300. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

