Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2
- PMID: 15840688
- PMCID: PMC1774880
- DOI: 10.1136/gut.2004.062794
Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2
Abstract
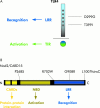
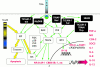
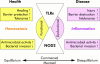
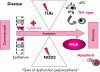
Toll-like receptors (TLR) and NOD2 are emerging as key mediators of innate host defence in the intestinal mucosa, crucially involved in maintaining mucosal as well as commensal homeostasis. Recent observations suggest new (patho-) physiological mechanisms of how functional versus dysfunctional TLRx/NOD2 pathways may oppose or favour inflammatory bowel disease (IBD). In health, TLRx signalling protects the intestinal epithelial barrier and confers commensal tolerance whereas NOD2 signalling exerts antimicrobial activity and prevents pathogenic invasion. In disease, aberrant TLRx and/or NOD2 signalling may stimulate diverse inflammatory responses leading to acute and chronic intestinal inflammation with many different clinical phenotypes.
Figures
References
-
- Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002;296:298–300. - PubMed
-
- Xu Y, Tao X, Shen B, et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 2000;408:111–15. - PubMed
-
- Cario E, Rosenberg IM, Brandwein SL, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 2000;164:966–72. - PubMed
-
- Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed tlr5 to induce epithelial proinflammatory gene expression. J Immunol 2001;167:1882–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
