Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion
- PMID: 15840721
- PMCID: PMC1087934
- DOI: 10.1073/pnas.0501519102
Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion
Abstract
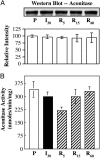
Prooxidents can induce reversible inhibition or irreversible inactivation and degradation of the mitochondrial enzyme aconitase. Cardiac ischemia/reperfusion is associated with an increase in mitochondrial free radical production. In the current study, the effects of reperfusion-induced production of prooxidants on mitochondrial aconitase and proteolytic activity were determined to assess whether alterations represented a regulated response to changes in redox status or oxidative damage. Evidence is provided that ATP-dependent proteolytic activity increased during early reperfusion followed by a time-dependent reduction in activity to control levels. These alterations in proteolytic activity paralleled an increase and subsequent decrease in the level of oxidatively modified protein. In vitro data supports a role for prooxidants in the activation of ATP-dependent proteolytic activity. Despite inhibition during early periods of reperfusion, aconitase was not degraded under the conditions of these experiments. Aconitase activity exhibited a decline in activity followed by reactivation during cardiac reperfusion. Loss and regain in activity involved reversible sulfhydryl modification. Aconitase was found to associate with the iron binding protein frataxin exclusively during reperfusion. In vitro, frataxin has been shown to protect aconitase from [4Fe-4S](2+) cluster disassembly, irreversible inactivation, and, potentially, degradation. Thus, the response of mitochondrial aconitase and ATP-dependent proteolytic activity to reperfusion-induced prooxidant production appears to be a regulated event that would be expected to reduce irreparable damage to the mitochondria.
Figures


Similar articles
-
Redox-dependent modulation of aconitase activity in intact mitochondria.Biochemistry. 2003 Dec 23;42(50):14846-55. doi: 10.1021/bi0353979. Biochemistry. 2003. PMID: 14674759
-
Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity.Science. 2004 Jul 9;305(5681):242-5. doi: 10.1126/science.1098991. Science. 2004. PMID: 15247478
-
Selective inactivation of redox-sensitive mitochondrial enzymes during cardiac reperfusion.Arch Biochem Biophys. 2002 Oct 15;406(2):222-8. doi: 10.1016/s0003-9861(02)00446-0. Arch Biochem Biophys. 2002. PMID: 12361710
-
Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species.Redox Rep. 2014 Jan;19(1):8-15. doi: 10.1179/1351000213Y.0000000073. Epub 2013 Nov 22. Redox Rep. 2014. PMID: 24266943 Free PMC article. Review.
-
Alterations in mitochondrial and cytosolic methionine sulfoxide reductase activity during cardiac ischemia and reperfusion.Exp Gerontol. 2006 Jul;41(7):663-7. doi: 10.1016/j.exger.2006.03.011. Epub 2006 May 4. Exp Gerontol. 2006. PMID: 16677789 Review.
Cited by
-
Antioxidant responses and cellular adjustments to oxidative stress.Redox Biol. 2015 Dec;6:183-197. doi: 10.1016/j.redox.2015.07.008. Epub 2015 Jul 21. Redox Biol. 2015. PMID: 26233704 Free PMC article. Review.
-
The Role of Lonp1 on Mitochondrial Functions during Cardiovascular and Muscular Diseases.Antioxidants (Basel). 2023 Feb 28;12(3):598. doi: 10.3390/antiox12030598. Antioxidants (Basel). 2023. PMID: 36978846 Free PMC article. Review.
-
Monomeric yeast frataxin is an iron-binding protein.Biochemistry. 2006 Jun 27;45(25):7767-77. doi: 10.1021/bi060424r. Biochemistry. 2006. PMID: 16784228 Free PMC article.
-
Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions.Redox Biol. 2013 Dec 19;2:123-39. doi: 10.1016/j.redox.2013.12.011. eCollection 2014. Redox Biol. 2013. PMID: 24455476 Free PMC article. Review.
-
Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging.Antioxid Redox Signal. 2010 Apr;12(4):503-35. doi: 10.1089/ars.2009.2598. Antioxid Redox Signal. 2010. PMID: 19650712 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

