Disulfide locked variants of factor VIIa with a restricted beta-strand conformation have enhanced enzymatic activity
- PMID: 15840825
- PMCID: PMC2253269
- DOI: 10.1110/ps.041097505
Disulfide locked variants of factor VIIa with a restricted beta-strand conformation have enhanced enzymatic activity
Abstract
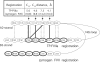
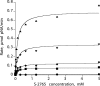
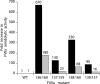
Proteolytic processing of zymogen Factor VII to Factor VIIa (FVIIa) is necessary but not sufficient for maximal proteolytic activity, which requires an additional allosteric influence induced upon binding to its cofactor tissue factor (TF). A key conformational change affecting the zymogenicity of FVIIa involves a unique three-residue shift in the position of beta-strand B2 in their zymogen and protease forms. By selectively introducing new disulfide bonds, we locked the conformation of these strands into an active TF*FVIIa-like state. FVIIa mutants designated 136:160, 137:159, 138:160, and 139:157, reflecting the position of the new disulfide bond (chymotypsinogen numbering), were expressed and purified by TF affinity chromatography. Mass spectrometric analysis of tryptic peptides from the FVIIa mutants confirmed the new disulfide bond formation. Kinetic analysis of amidolytic activity revealed that all FVIIa variants alone had increased specific activity compared to wild type, the largest being for variants 136:160 and 138:160 with substrate S-2765, having 670- and 330-fold increases, respectively. Notably, FVIIa disulfide-locked variants no longer required TF as a cofactor for maximal activity in amidolytic assays. In the presence of soluble TF, activity was enhanced 20- and 12-fold for variants 136:160 and 138:160, respectively, compared to wild type. With relipidated TF, mutants 136:160 and 137:159 also had an approximate threefold increase in their V(max)/K(m) values for FX activation but no significant improvement in TF-dependent clotting assays. Thus, while large rate enhancements were obtained for amidolytic substrates binding at the active site, macro-molecular substrates that bind to FVIIa exosites entail more complex catalytic requirements.
Figures


Similar articles
-
Prevention of beta strand movement into a zymogen-like position does not confer higher activity to coagulation factor VIIa.Biochemistry. 2004 Nov 9;43(44):14096-103. doi: 10.1021/bi048721o. Biochemistry. 2004. PMID: 15518559
-
Activation loop 3 and the 170 loop interact in the active conformation of coagulation factor VIIa.FEBS J. 2009 Jun;276(11):3099-109. doi: 10.1111/j.1742-4658.2009.07028.x. Epub 2009 Apr 23. FEBS J. 2009. PMID: 19490111
-
Substitution of aspartic acid for methionine-306 in factor VIIa abolishes the allosteric linkage between the active site and the binding interface with tissue factor.Biochemistry. 2001 Mar 20;40(11):3251-6. doi: 10.1021/bi001612z. Biochemistry. 2001. PMID: 11258943
-
Interaction of activated factor VII and active site-inhibited activated factor VII with tissue factor.Blood Coagul Fibrinolysis. 1998 Mar;9 Suppl 1:S67-71. Blood Coagul Fibrinolysis. 1998. PMID: 9819031 Review.
-
Allosteric activation of coagulation factor VIIa.Front Biosci (Landmark Ed). 2011 Jun 1;16(8):3156-63. doi: 10.2741/3903. Front Biosci (Landmark Ed). 2011. PMID: 21622226 Review.
Cited by
-
Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1.Mol Cell Biol. 2009 Apr;29(8):2181-92. doi: 10.1128/MCB.01517-08. Epub 2009 Feb 2. Mol Cell Biol. 2009. PMID: 19188440 Free PMC article.
-
ARNT PAS-B has a fragile native state structure with an alternative beta-sheet register nearby in sequence space.Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2617-22. doi: 10.1073/pnas.0808270106. Epub 2009 Feb 5. Proc Natl Acad Sci U S A. 2009. PMID: 19196990 Free PMC article.
References
-
- Banner, D.W., D’Arcy, A., Chène, C., Winkler, F.K., Guha, A., Konigsberg, W.H., Nemerson, Y., and Kirchhofer, D. 1996. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature 380 41–46. - PubMed
-
- Boose, J.A., Kuismanen, E., Gerard, R., Sambrook, J., and Gething, M.-J. 1989. The single-chain form of tissue-type plasminogen activator has catalytic activity: Studies with a mutant enzyme that lacks the cleavage site. Biochemistry 28 635–643. - PubMed
-
- Broze, Jr., G.J. 1992. Tissue factor pathway inhibitor and the revised hypothesis of blood coagulation. Trends Cardiovasc. Med. 2 72–77. - PubMed
-
- Butenas, S. and Mann, K.G. 2003. Response: Mechanism of action of high-dose factor VIIa. Arterioscler. Thromb. Vasc. Biol. 23 10. - PubMed
-
- Butenas, S., Ribarik, N., and Mann, K.G. 1993. Synthetic substrates for human factor VIIa and factor VIIa-tissue factor. Biochemistry 32 6531–6538. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

