Immunopathogenesis and therapy of cutaneous T cell lymphoma
- PMID: 15841167
- PMCID: PMC1070436
- DOI: 10.1172/JCI24826
Immunopathogenesis and therapy of cutaneous T cell lymphoma
Erratum in
- J Clin Invest. 2007 Mar;117(3):836
Abstract
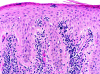
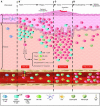
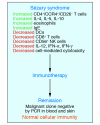
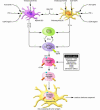
Cutaneous T cell lymphomas (CTCLs) are a heterogenous group of lymphoproliferative disorders caused by clonally derived, skin-invasive T cells. Mycosis fungoides (MF) and Sezary syndrome (SS) are the most common types of CTCLs and are characterized by malignant CD4(+)/CLA(+)/CCR4(+) T cells that also lack the usual T cell surface markers CD7 and/or CD26. As MF/SS advances, the clonal dominance of the malignant cells results in the expression of predominantly Th2 cytokines, progressive immune dysregulation in patients, and further tumor cell growth. This review summarizes recent insights into the pathogenesis and immunobiology of MF/SS and how these have shaped current therapeutic approaches, in particular the growing emphasis on enhancement of host antitumor immune responses as the key to successful therapy.
Figures



References
-
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N. Engl. J. Med. 2004;350:1978–1988. - PubMed
-
- Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J. Eur. Acad. Dermatol. Venereol. 2004;18:397–415. - PubMed
-
- Willemze, R., et al. 2005. WHO-EORTC classification for cutaneous lymphomas. Blood. doi:10.1182/blood-2004-09-3502. - PubMed
-
- Leboit, P.E., and McCalmont, T.H. 1997. Cutaneous lymphomas and leukemias. In Lever’s histopathology of the skin. D. Elder, C. Jaworsky, and B. Johnson, editors. Lippincott-Raven. Philadelphia, Pennsylvania, USA. 820 pp.
-
- Kim YH, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch. Dermatol. 2003;139:857–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

