Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen
- PMID: 15841176
- PMCID: PMC1070403
- DOI: 10.1172/JCI21386
Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen
Abstract
Epidermolysis bullosa acquisita (EBA) is a subepidermal blistering disorder associated with tissue-bound and circulating autoantibodies specific to type VII collagen, a major constituent of the dermal-epidermal junction. Previous attempts to transfer the disease by injection of patient autoantibodies into mice have been unsuccessful. To study the pathogenic relevance of antibodies specific to type VII collagen in vivo, we generated and characterized rabbit antibodies specific to a murine form of this antigen and passively transferred them into adult nude, BALB/c, and C57BL/6 mice. Immune rabbit IgG bound to the lamina densa of murine skin and immunoblotted type VII collagen. Mice injected with purified IgG specific to type VII collagen, in contrast to control mice, developed subepidermal skin blisters, reproducing the human disease at the clinical, histological, electron microscopical, and immunopathological levels. Titers of rabbit IgG in the serum of mice correlated with the extent of the disease. F(ab')(2) fragments of rabbit IgG specific to type VII collagen were not pathogenic. When injected into C5-deficient mice, antibodies specific to type VII collagen failed to induce the disease, whereas C5-sufficient mice were susceptible to blister induction. This animal model for EBA should facilitate further dissection of the pathogenesis of this disease and development of new therapeutic strategies.
Figures


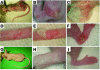
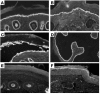

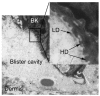
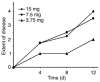

Comment in
-
The pathophysiology of autoimmune blistering diseases.J Clin Invest. 2005 Apr;115(4):825-8. doi: 10.1172/JCI24855. J Clin Invest. 2005. PMID: 15841169 Free PMC article.
References
-
- Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited) Immunol. Today. 1993;14:426–430. - PubMed
-
- Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv. Dermatol. 2000;16:113–157. - PubMed
-
- Woodley DT, et al. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N. Engl. J. Med. 1984;310:1007–1013. - PubMed
-
- Stanley JR, Rubinstein N, Klaus-Kovtun V. Epidermolysis bullosa acquisita antigen is synthesized by both human keratinocytes and human dermal fibroblasts. J. Invest. Dermatol. 1985;85:542–545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

