Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display
- PMID: 15841178
- PMCID: PMC1070425
- DOI: 10.1172/JCI24185
Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display
Abstract
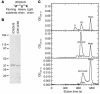
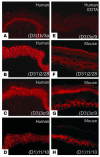
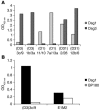
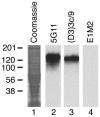
Pemphigus is a life-threatening blistering disorder of the skin and mucous membranes caused by pathogenic autoantibodies to desmosomal adhesion proteins desmoglein 3 (Dsg3) and Dsg1. Mechanisms of antibody pathogenicity are difficult to characterize using polyclonal patient sera. Using antibody phage display, we have isolated repertoires of human anti-Dsg mAbs as single-chain variable-region fragments (scFvs) from a patient with active mucocutaneous pemphigus vulgaris. ScFv mAbs demonstrated binding to Dsg3 or Dsg1 alone, or both Dsg3 and Dsg1. Inhibition ELISA showed that the epitopes defined by these scFvs are blocked by autoantibodies from multiple pemphigus patients. Injection of scFvs into neonatal mice identified 2 pathogenic scFvs that caused blisters histologically similar to those observed in pemphigus patients. Similarly, these 2 scFvs, but not others, induced cell sheet dissociation of cultured human keratinocytes, indicating that both pathogenic and nonpathogenic antibodies were isolated. Genetic analysis of these mAbs showed restricted patterns of heavy and light chain gene usage, which were distinct for scFvs with different desmoglein-binding specificities. Detailed characterization of these pemphigus mAbs should lead to a better understanding of the immunopathogenesis of disease and to more specifically targeted therapeutic approaches.
Figures





Comment in
-
The pathophysiology of autoimmune blistering diseases.J Clin Invest. 2005 Apr;115(4):825-8. doi: 10.1172/JCI24855. J Clin Invest. 2005. PMID: 15841169 Free PMC article.
References
-
- Payne AS, Hanakawa Y, Amagai M, Stanley JR. Desmosomes and disease: pemphigus and bullous impetigo. Curr. Opin. Cell Biol. 2004;16:536–543. - PubMed
-
- Ishii K, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J. Immunol. 1997;159:2010–2017. - PubMed
-
- Ding X, et al. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J. Invest. Dermatol. 1997;109:592–596. - PubMed
-
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J. Am. Acad. Dermatol. 1999;40:167–170. - PubMed
-
- Miyagawa S, et al. Late development of antidesmoglein 1 antibodies in pemphigus vulgaris: correlation with disease progression. Br. J. Dermatol. 1999;141:1084–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

