The role of insulin receptor substrate 2 in hypothalamic and beta cell function
- PMID: 15841180
- PMCID: PMC1069106
- DOI: 10.1172/JCI24445
The role of insulin receptor substrate 2 in hypothalamic and beta cell function
Abstract
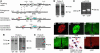
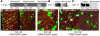
Insulin receptor substrate 2 (Irs2) plays complex roles in energy homeostasis. We generated mice lacking Irs2 in beta cells and a population of hypothalamic neurons (RIPCreIrs2KO), in all neurons (NesCreIrs2KO), and in proopiomelanocortin neurons (POMCCreIrs2KO) to determine the role of Irs2 in the CNS and beta cell. RIPCreIrs2KO mice displayed impaired glucose tolerance and reduced beta cell mass. Overt diabetes did not ensue, because beta cells escaping Cre-mediated recombination progressively populated islets. RIPCreIrs2KO and NesCreIrs2KO mice displayed hyperphagia, obesity, and increased body length, which suggests altered melanocortin action. POMCCreIrs2KO mice did not display this phenotype. RIPCreIrs2KO and NesCreIrs2KO mice retained leptin sensitivity, which suggests that CNS Irs2 pathways are not required for leptin action. NesCreIrs2KO and POMCCreIrs2KO mice did not display reduced beta cell mass, but NesCreIrs2KO mice displayed mild abnormalities of glucose homeostasis. RIPCre neurons did not express POMC or neuropeptide Y. Insulin and a melanocortin agonist depolarized RIPCre neurons, whereas leptin was ineffective. Insulin hyperpolarized and leptin depolarized POMC neurons. Our findings demonstrate a critical role for IRS2 in beta cell and hypothalamic function and provide insights into the role of RIPCre neurons, a distinct hypothalamic neuronal population, in growth and energy homeostasis.
Figures
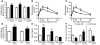




References
-
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5:177–268.
-
- Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. - PubMed
-
- Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. - PubMed
-
- Bruning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. - PubMed
-
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002;5:566–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

