Ikaros integrates endocrine and immune system development
- PMID: 15841184
- PMCID: PMC1070405
- DOI: 10.1172/JCI22486
Ikaros integrates endocrine and immune system development
Abstract
Ikaros transcription factors are essential regulators of lymphopoiesis and the development of the immune system. We now show that Ikaros is expressed in hormone-producing pituitary corticomelanotroph cells, where it binds the proopiomelanocortin promoter and regulates endogenous gene expression. Loss of Ikaros in vivo results in contraction of the pituitary corticomelanotroph population, reduced circulating adrenocorticotrophic hormone levels, and adrenal glucocorticoid insufficiency. While hemopoietic reconstitution failed to correct this hormonal deficit, the phenotype of reduced body weight and diminished survival was rescued by systemic glucocorticoid-hormone administration. Given the established immunomodulatory properties of glucocorticoid hormones, these findings reveal a novel role for Ikaros in orchestrating immune-endocrine development and function.
Figures
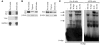
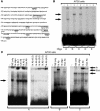
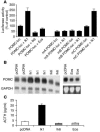

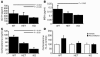

Comment in
-
Ikaros transcription factors: flying between stress and inflammation.J Clin Invest. 2005 Apr;115(4):844-8. doi: 10.1172/JCI24886. J Clin Invest. 2005. PMID: 15841175 Free PMC article.
References
-
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995;332:1351–1362. - PubMed
-
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 1999;79:1–71. - PubMed
-
- Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr. Rev. 1998;19:798–827. - PubMed
-
- Asa SL, Ezzat S. Molecular determinants of pituitary cytodifferentiation. Pituitary. 1999;1:159–168. - PubMed
-
- Molnar A, et al. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J. Immunol. 1996;156:585–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

