Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner
- PMID: 15841212
- PMCID: PMC1070637
- DOI: 10.1172/JCI23371
Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner
Erratum in
- J Clin Invest. 2012 Feb 1;122(2):781
Abstract
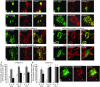
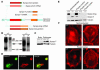
Synaptopodin is the founding member of a novel class of proline-rich actin-associated proteins highly expressed in telencephalic dendrites and renal podocytes. Synaptopodin-deficient (synpo(-/-)) mice lack the dendritic spine apparatus and display impaired activity-dependent long-term synaptic plasticity. In contrast, the ultrastructure of podocytes in synpo(-/-) mice is normal. Here we show that synpo(-/-) mice display impaired recovery from protamine sulfate-induced podocyte foot process (FP) effacement and LPS-induced nephrotic syndrome. Similarly, synpo(-/-) podocytes show impaired actin filament reformation in vitro. We further demonstrate that synaptopodin exists in 3 isoforms, neuronal Synpo-short (685 AA), renal Synpo-long (903 AA), and Synpo-T (181 AA). The C terminus of Synpo-long is identical to that of Synpo-T. All 3 isoforms specifically interact with alpha-actinin and elongate alpha-actinin-induced actin filaments. synpo(-/-) mice lack Synpo-short and Synpo-long expression but show an upregulation of Synpo-T protein expression in podocytes, though not in the brain. Gene silencing of Synpo-T abrogates stress-fiber formation in synpo(-/-) podocytes, demonstrating that Synpo-T serves as a backup for Synpo-long in synpo(-/-) podocytes. In concert, synaptopodin regulates the actin-bundling activity of alpha-actinin in highly dynamic cell compartments, such as podocyte FPs and the dendritic spine apparatus.
Figures





References
-
- Mundel P, Gilbert P, Kriz W. Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J. Histochem. Cytochem. 1991;39:1047–1056. - PubMed
-
- Deller T, Merten T, Roth SU, Mundel P, Frotscher M. Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J. Comp. Neurol. 2000;418:164–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

