Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks
- PMID: 15843448
- PMCID: PMC1755272
- DOI: 10.1136/ard.2004.035105
Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks
Abstract
Objective: To evaluate the continued safety and durability of clinical response in patients with ankylosing spondylitis receiving etanercept.
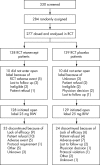
Methods: 277 patients who had participated in a previous randomised, double blind, placebo controlled 24 week trial were eligible to continue in this open label extension study. All patients who enrolled in the open label extension (n = 257) received subcutaneous etanercept 25 mg twice weekly for up to 72 weeks, for a combined 96 weeks of cumulative trial and open label experience. For the patients who had received etanercept for 24 weeks in the double blind trial, this represented almost 2 years of continuous etanercept treatment.
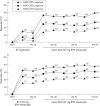
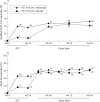
Results: Patients continuing etanercept treatment had a sustained response for almost 2 years, with 74% achieving an ASsessments in Ankylosing Spondylitis 20% (ASAS 20) response after 96 weeks of etanercept treatment. Patients who had received placebo in the preceding double blind trial had similar responses, with 70% of patients attaining an ASAS 20 response after 24 weeks of etanercept treatment and 78% achieving an ASAS 20 response after 72 weeks. Improved spinal mobility was seen in both groups. Etanercept was well tolerated in patients treated for up to 96 weeks.
Conclusion: The subcutaneous administration of twice weekly doses of etanercept provided sustained durability of response in the improvement of signs and symptoms of ankylosing spondylitis for nearly 2 years.
Figures
Similar articles
-
Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis.Ann Rheum Dis. 2004 Dec;63(12):1594-600. doi: 10.1136/ard.2004.020875. Epub 2004 Sep 2. Ann Rheum Dis. 2004. PMID: 15345498 Free PMC article. Clinical Trial.
-
Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial.Arthritis Rheum. 2003 Nov;48(11):3230-6. doi: 10.1002/art.11325. Arthritis Rheum. 2003. PMID: 14613288 Clinical Trial.
-
Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis.Ann Rheum Dis. 2008 Mar;67(3):346-52. doi: 10.1136/ard.2007.078139. Epub 2007 Oct 29. Ann Rheum Dis. 2008. PMID: 17967833 Clinical Trial.
-
Etanercept: in ankylosing spondylitis.BioDrugs. 2004;18(3):199-205; discussion 206. doi: 10.2165/00063030-200418030-00006. BioDrugs. 2004. PMID: 15161337 Review.
-
Etanercept in the treatment of ankylosing spondylitis: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials, and the comparison of the Caucasian and Chinese population.Eur J Orthop Surg Traumatol. 2013 Jul;23(5):497-506. doi: 10.1007/s00590-012-1035-7. Epub 2012 Jun 29. Eur J Orthop Surg Traumatol. 2013. PMID: 23412168 Review.
Cited by
-
Maintenance of improvement in spinal mobility, physical function and quality of life in patients with ankylosing spondylitis after 5 years in a clinical trial of adalimumab.Rheumatology (Oxford). 2015 Jul;54(7):1210-9. doi: 10.1093/rheumatology/keu438. Epub 2014 Dec 25. Rheumatology (Oxford). 2015. PMID: 25541333 Free PMC article. Clinical Trial.
-
Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: a case-control study.Arthritis Res Ther. 2012 Oct 11;14(5):R215. doi: 10.1186/ar4054. Arthritis Res Ther. 2012. PMID: 23058191 Free PMC article.
-
New advances in the understanding and treatment of axial spondyloarthritis: from chance to choice.Ther Adv Chronic Dis. 2018 Mar;9(3):77-87. doi: 10.1177/2040622317743486. Epub 2017 Dec 14. Ther Adv Chronic Dis. 2018. PMID: 29511503 Free PMC article. Review.
-
Inflammatory Bowel Disease-related Spondyloarthritis: The Last Unexplored Territory of Rheumatology.Mediterr J Rheumatol. 2022 Apr 15;33(Suppl 1):126-136. doi: 10.31138/mjr.33.1.126. eCollection 2022 Mar. Mediterr J Rheumatol. 2022. PMID: 36127923 Free PMC article. Review.
-
Low doses of etanercept can be effective in ankylosing spondylitis patients who achieve remission of the disease.Clin Rheumatol. 2011 Jul;30(7):993-6. doi: 10.1007/s10067-011-1722-5. Epub 2011 Mar 4. Clin Rheumatol. 2011. PMID: 21373780
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

