Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation
- PMID: 15843458
- PMCID: PMC556276
- DOI: 10.1073/pnas.0502088102
Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation
Abstract
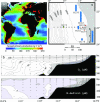
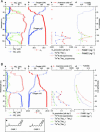
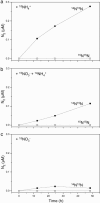
In many oceanic regions, growth of phytoplankton is nitrogen-limited because fixation of N2 cannot make up for the removal of fixed inorganic nitrogen (NH4+, NO2-, and NO3-) by anaerobic microbial processes. Globally, 30-50% of the total nitrogen loss occurs in oxygen-minimum zones (OMZs) and is commonly attributed to denitrification (reduction of nitrate to N2 by heterotrophic bacteria). Here, we show that instead, the anammox process (the anaerobic oxidation of ammonium by nitrite to yield N2) is mainly responsible for nitrogen loss in the OMZ waters of one of the most productive regions of the world ocean, the Benguela upwelling system. Our in situ experiments indicate that nitrate is not directly converted to N2 by heterotrophic denitrification in the suboxic zone. In the Benguela system, nutrient profiles, anammox rates, abundances of anammox cells, and specific biomarker lipids indicate that anammox bacteria are responsible for massive losses of fixed nitrogen. We have identified and directly linked anammox bacteria to the removal of fixed inorganic nitrogen in the OMZ waters of an open-ocean setting. We hypothesize that anammox could also be responsible for substantial nitrogen loss from other OMZ waters of the ocean.
Figures


References
-
- Copin-Montegut, C. & Copin-Montegut, G. (1983) Deep-Sea Res. 30, 31-46.
-
- Toggweiler, J. R. (1999) Nature 400, 511-512.
-
- Redfield, A. C., Ketchum, B. H. & Richards, F. A. (1963) in The Sea, ed. Hill, M. N. (Interscience, New York), Vol. 2, pp. 26-77.
-
- Devol, A. H. (2003) Nature 422, 575-576. - PubMed
-
- Richards, F. A. (1965) in Chemical Oceanography, eds. Ripley, J. P. & Skirrow, G. (Academic, London), pp. 611-645.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

