TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells
- PMID: 15843517
- PMCID: PMC2562293
- DOI: 10.4049/jimmunol.174.9.5215
TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells
Abstract
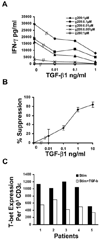
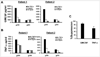
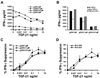
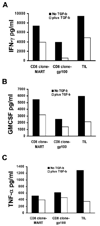
TGF-beta1 is a potent immunoregulatory cytokine. However, its impact on the generation and effector function of Ag-specific human effector memory CD8 T cells had not been evaluated. Using Ag-specific CD8 T cells derived from melanoma patients immunized with the gp100 melanoma Ag, we demonstrate that the addition of TGF-beta1 to the initial Ag activation cultures attenuated the gain of effector function by Ag-specific memory CD8 T cells while the phenotypic changes associated with activation and differentiation into effector memory were comparable to control cultures. These activated memory CD8 T cells consistently expressed lower mRNA levels for T-bet, suggesting a mechanism for TGF-beta1-mediated suppression of gain of effector function in memory T cells. Moreover, TGF-beta1 induced a modest expression of CCR7 on Ag-activated memory CD8 T cells. TGF-beta1 also suppressed cytokine secretion by Ag-specific effector memory CD8 T cells, as well as melanoma-reactive tumor-infiltrating lymphocytes and CD8 T cell clones. These results demonstrate that TGF-beta1 suppresses not only the acquisition but also expression of effector function on human memory CD8 T cells and tumor-infiltrating lymphocytes reactive against melanoma, suggesting that TGF-beta1-mediated suppression can hinder the therapeutic benefits of vaccination, as well as immunotherapy in cancer patients.
Figures

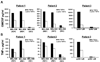

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

