The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors
- PMID: 15843601
- PMCID: PMC4461238
- DOI: 10.1523/JNEUROSCI.4631-04.2005
The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors
Abstract
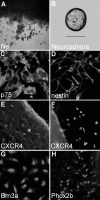
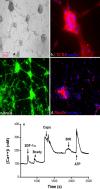
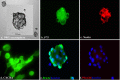
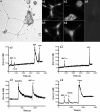
Chemokines and their receptors are essential for the development and organization of the hematopoietic/lymphopoietic system and have now been shown to be expressed by different types of cells in the nervous system. In mouse embryos, we observed expression of the chemokine (CXC motif) receptor 4 (CXCR4) by neural crest cells migrating from the dorsal neural tube and in the dorsal root ganglia (DRGs). Stromal cell-derived factor-1 (SDF-1), the unique agonist for CXCR4, was expressed along the path taken by crest cells to the DRGs, suggesting that SDF-1/CXCR4 signaling is needed for their migration. CXCR4 null mice exhibited small and malformed DRGs. Delayed migration to the DRGs was suggested by ectopic cells expressing tyrosine receptor kinase A (TrkA) and TrkC, neurotrophin receptors required by DRG sensory neuron development. In vitro, the CXCR4 chemokine receptor was upregulated by migratory progenitor cells just as they exited mouse neural tube explants, and SDF-1 acted as a chemoattractant for these cells. Most CXCR4-expressing progenitors differentiated to form sensory neurons with the properties of polymodal nociceptors. Furthermore, DRGs contained a population of progenitor cells that expressed CXCR4 receptors in vitro and differentiated into neurons with a similar phenotype. Our findings indicate an important role for SDF-1/CXCR4 signaling in directing the migration of sensory neuron progenitors to the DRG and potentially in other aspects of development once the DRGs have coalesced.
Figures





References
-
- Agarwala S, Sanders TA, Ragsdale CW (2001) Sonic hedgehog control of size and shape in midbrain pattern formation. Science 291: 2147-2150. - PubMed
-
- An M, Luo R, Henion PD (2002) Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J Comp Neurol 446: 267-275. - PubMed
-
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ (2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249-4260. - PubMed
-
- Bodner A, Toth PT, Miller RJ (2004) Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol 118: 246-253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
