Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress
- PMID: 15843609
- PMCID: PMC6724949
- DOI: 10.1523/JNEUROSCI.0122-05.2005
Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress
Abstract
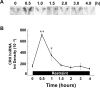
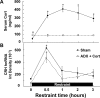
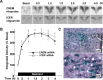
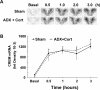
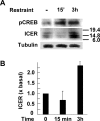
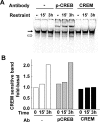
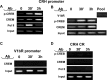
The role of glucocorticoids and the repressor isoform of cAMP response element (CRE) modulator (CREM), inducible cAMP early repressor (ICER), in limiting corticotropin-releasing hormone (CRH) transcription during restraint stress were examined in both intact and adrenalectomized rats receiving glucocorticoid replacement. CRH primary transcript, measured by intronic in situ hybridization, increased after 30 min of restraint and returned to basal levels by 90 min, despite the persistent stressor. The decline was independent of circulating glucocorticoids, because adrenalectomized rats displayed an identical pattern. ICER mRNA in the hypothalamic paraventricular nucleus (PVN) increased after 30 min and remained elevated for up to 4 h in a glucocorticoid-independent manner. Western blot and electrophoretic mobility shift assay analyses showed increases in endogenous ICER in the PVN of rats subjected to restraint stress for 3 h. Chromatin immunoprecipitation assays showed the recruitment of CREM by the CRH CRE in conjunction with decreases in RNA polymerase II (Pol II) binding in the PVN region of rats restrained for 3 h. These data show that stress-induced glucocorticoids do not mediate the limitation of CRH transcription. Furthermore, the ability of CREM to bind the CRH CRE and the time relationship between elevated CREM and reduced Pol II recruitment by the CRH promoter suggest that inhibitory isoforms of CREM induced during stress contribute to the decline in CRH gene transcription during persistent stimulation.
Figures
Similar articles
-
Inhibition of corticotrophin-releasing hormone transcription by inducible cAMP-early repressor in the hypothalamic cell line, 4B.J Neuroendocrinol. 2006 Jan;18(1):42-9. doi: 10.1111/j.1365-2826.2005.01383.x. J Neuroendocrinol. 2006. PMID: 16451219
-
Expression of type 1 corticotropin-releasing hormone (CRH) receptor mRNA in the hypothalamic paraventricular nucleus following restraint stress in CRH-deficient mice.Brain Res. 2005 Jun 28;1048(1-2):131-7. doi: 10.1016/j.brainres.2005.04.065. Brain Res. 2005. PMID: 15919058
-
Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids.Mol Endocrinol. 2013 Nov;27(11):1796-807. doi: 10.1210/me.2013-1095. Epub 2013 Sep 24. Mol Endocrinol. 2013. PMID: 24065704 Free PMC article.
-
CRH: the link between hormonal-, metabolic- and behavioral responses to stress.J Chem Neuroanat. 2013 Dec;54:25-33. doi: 10.1016/j.jchemneu.2013.05.003. Epub 2013 Jun 15. J Chem Neuroanat. 2013. PMID: 23774011 Review.
-
Inducible cAMP early repressor (ICER) and brain functions.Mol Neurobiol. 2009 Aug;40(1):73-86. doi: 10.1007/s12035-009-8072-1. Epub 2009 May 13. Mol Neurobiol. 2009. PMID: 19434522 Free PMC article. Review.
Cited by
-
Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids.Proc Natl Acad Sci U S A. 2009 May 12;106(19):8038-42. doi: 10.1073/pnas.0812062106. Epub 2009 Apr 29. Proc Natl Acad Sci U S A. 2009. PMID: 19416907 Free PMC article.
-
Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription.Endocrinology. 2010 Mar;151(3):1109-18. doi: 10.1210/en.2009-0963. Epub 2010 Jan 15. Endocrinology. 2010. PMID: 20080871 Free PMC article.
-
Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation.J Neurosci. 2008 Jun 25;28(26):6642-51. doi: 10.1523/JNEUROSCI.1336-08.2008. J Neurosci. 2008. PMID: 18579737 Free PMC article.
-
Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat.Stress. 2009 Sep;12(5):400-11. doi: 10.1080/10253890802530730. Stress. 2009. PMID: 19065454 Free PMC article.
-
Hyperactivity of Hypothalamic-Pituitary-Adrenal Axis Due to Dysfunction of the Hypothalamic Glucocorticoid Receptor in Sigma-1 Receptor Knockout Mice.Front Mol Neurosci. 2017 Sep 6;10:287. doi: 10.3389/fnmol.2017.00287. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28932185 Free PMC article.
References
-
- Aarts MM, Tymianski M (2003) Novel treatment of excitotoxicity: targeted disruption of intracellular signalling from glutamate receptors. Biochem Pharmacol 66: 877-886. - PubMed
-
- Aguilera G (1994) Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 15: 321-350. - PubMed
-
- Aguilera G, Kiss A (1993) Activation of magnocellular vasopressin responses to non-osmotic stress after chronic adrenal demedullation in rats. J Neuroendocrinol 5: 501-507. - PubMed
-
- Aguilera G, Harwood JP, Wilson JX, Morell J, Brown JH, Catt KJ (1983) Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem 258: 8039-8045. - PubMed
-
- Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ (2004) Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur J Neurosci 19: 777-782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
