Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation
- PMID: 15843611
- PMCID: PMC6724965
- DOI: 10.1523/JNEUROSCI.4312-04.2005
Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation
Abstract
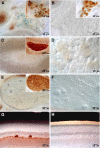
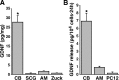
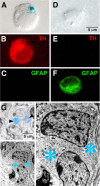
Glial cell line-derived neurotrophic factor (GDNF) exerts a notable protective effect on dopaminergic neurons in rodent and primate models of Parkinson's disease (PD). The clinical applicability of this therapy is, however, hampered by the need of a durable and stable GDNF source allowing the safe and continuous delivery of the trophic factor into the brain parenchyma. Intrastriatal carotid body (CB) autografting is a neuroprotective therapy potentially useful in PD. It induces long-term recovery of parkinsonian animals through a trophic effect on nigrostriatal neurons and causes amelioration of symptoms in some PD patients. Moreover, the adult rodent CB has been shown to express GDNF. Here we show, using heterozygous GDNF/lacZ knock-out mice, that unexpectedly CB dopaminergic glomus, or type I, cells are the source of CB GDNF. Among the neural or paraneural cells tested, glomus cells are those that synthesize and release the highest amount of GDNF in the adult rodent (as measured by standard and in situ ELISA). Furthermore, GDNF expression by glomus cells is maintained after intrastriatal grafting and in CB of aged and parkinsonian 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated animals. Thus, glomus cells appear to be prototypical abundant sources of GDNF, ideally suited to be used as biological pumps for the endogenous delivery of trophic factors in PD and other neurodegenerative diseases.
Figures



Similar articles
-
The neurogenic niche in the carotid body and its applicability to antiparkinsonian cell therapy.J Neural Transm (Vienna). 2009 Aug;116(8):975-82. doi: 10.1007/s00702-009-0201-5. Epub 2009 Mar 5. J Neural Transm (Vienna). 2009. PMID: 19263191 Review.
-
Protection and Repair of the Nigrostriatal Pathway with Stem-Cell-Derived Carotid Body Glomus Cell Transplants in Chronic MPTP Parkinsonian Model.Int J Mol Sci. 2023 Mar 14;24(6):5575. doi: 10.3390/ijms24065575. Int J Mol Sci. 2023. PMID: 36982650 Free PMC article.
-
Continuous exposure to glial cell line-derived neurotrophic factor to mature dopaminergic transplants impairs the graft's ability to improve spontaneous motor behavior in parkinsonian rats.Neuroscience. 2006 Aug 11;141(1):521-31. doi: 10.1016/j.neuroscience.2006.03.068. Epub 2006 May 11. Neuroscience. 2006. PMID: 16697115
-
Connexin 30 deficiency attenuates A2 astrocyte responses and induces severe neurodegeneration in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride Parkinson's disease animal model.J Neuroinflammation. 2018 Aug 13;15(1):227. doi: 10.1186/s12974-018-1251-0. J Neuroinflammation. 2018. PMID: 30103794 Free PMC article.
-
Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review.
Cited by
-
Differential impairment of catecholaminergic cell maturation and survival by genetic mitochondrial complex II dysfunction.Mol Cell Biol. 2012 Aug;32(16):3347-57. doi: 10.1128/MCB.00128-12. Epub 2012 Jun 18. Mol Cell Biol. 2012. PMID: 22711987 Free PMC article.
-
Combined cell-based therapy strategies for the treatment of Parkinson's disease: focus on mesenchymal stromal cells.Neural Regen Res. 2023 Mar;18(3):478-484. doi: 10.4103/1673-5374.350193. Neural Regen Res. 2023. PMID: 36018150 Free PMC article. Review.
-
The neurogenic niche in the carotid body and its applicability to antiparkinsonian cell therapy.J Neural Transm (Vienna). 2009 Aug;116(8):975-82. doi: 10.1007/s00702-009-0201-5. Epub 2009 Mar 5. J Neural Transm (Vienna). 2009. PMID: 19263191 Review.
-
Modelling of a targeted nanotherapeutic 'stroma' to deliver the cytokine LIF, or XAV939, a potent inhibitor of Wnt-β-catenin signalling, for use in human fetal dopaminergic grafts in Parkinson's disease.Dis Model Mech. 2014 Oct;7(10):1193-203. doi: 10.1242/dmm.015859. Epub 2014 Aug 1. Dis Model Mech. 2014. PMID: 25085990 Free PMC article.
-
Cellular basis of learning and memory in the carotid body.Front Synaptic Neurosci. 2022 Aug 15;14:902319. doi: 10.3389/fnsyn.2022.902319. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36046221 Free PMC article. Review.
References
-
- Abe K, Hayashi T (1997) Expression of the glial cell line-derived neurotrophic factor gene in rat brain after transient MCA occlusion. Brain Res 776: 230-234. - PubMed
-
- Alberch J, Perez-Navarro E, Canals JM (2002) Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull 57: 817-822. - PubMed
-
- Araujo DM, Hilt DC (1997) Glial cell line-derived neurotrophic factor attenuates the excitotoxin-induced behavioral and neurochemical deficits in a rodent model of Huntington's disease. Neuroscience 81: 1099-1110. - PubMed
-
- Arjona V, Minguez-Castellanos A, Montoro RJ, Ortega A, Escamilla F, Toledo-Aral JJ, Pardal R, Mendez-Ferrer S, Martin JM, Perez M, Katati MJ, Valencia E, Garcia T, Lopez-Barneo J (2003) Autotransplantation of human carotid body cell aggregates for treatment of Parkinson's disease. Neurosurgery 53: 321-330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
