HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy
- PMID: 15843809
- PMCID: PMC1242112
- DOI: 10.1038/sj.gt.3302533
HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy
Abstract
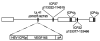
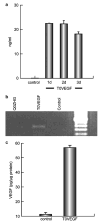
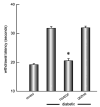
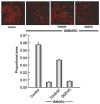
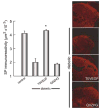
We examined the utility of herpes simplex virus (HSV) vector-mediated gene transfer of vascular endothelial growth factor (VEGF) in a mouse model of diabetic neuropathy. A replication-incompetent HSV vector with VEGF under the control of the HSV ICP0 promoter (vector T0VEGF) was constructed. T0VEGF expressed and released VEGF from primary dorsal root ganglion (DRG) neurons in vitro, and following subcutaneous inoculation in the foot, expressed VEGF in DRG and nerve in vivo. At 2 weeks after induction of diabetes, subcutaneous inoculation of T0VEGF prevented the reduction in sensory nerve amplitude characteristic of diabetic neuropathy measured 4 weeks later, preserved autonomic function measured by pilocarpine-induced sweating, and prevented the loss of nerve fibers in the skin and reduction of neuropeptide calcitonin gene-related peptide and substance P in DRG neurons of the diabetic mice. HSV-mediated transfer of VEGF to DRG may prove useful in treatment of diabetic neuropathy.
Figures



Comment in
-
Neural gene therapy: sensational finding.Gene Ther. 2005 Aug;12(15):1161-2. doi: 10.1038/sj.gt.3302560. Gene Ther. 2005. PMID: 15920461 No abstract available.
Similar articles
-
Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons.Brain. 2009 Apr;132(Pt 4):879-88. doi: 10.1093/brain/awp014. Epub 2009 Feb 24. Brain. 2009. PMID: 19244253 Free PMC article.
-
Decrease in neuroimmune activation by HSV-mediated gene transfer of TNFα soluble receptor alleviates pain in rats with diabetic neuropathy.Brain Behav Immun. 2014 Oct;41:144-51. doi: 10.1016/j.bbi.2014.05.009. Epub 2014 May 29. Brain Behav Immun. 2014. PMID: 24880032 Free PMC article.
-
Prevention of diabetic neuropathy by regulatable expression of HSV-mediated erythropoietin.Mol Ther. 2011 Feb;19(2):310-7. doi: 10.1038/mt.2010.215. Epub 2010 Oct 5. Mol Ther. 2011. PMID: 20924361 Free PMC article.
-
Exploiting the neurotherapeutic potential of peptides: targeted delivery using HSV vectors.Expert Opin Biol Ther. 2003 Dec;3(8):1233-9. doi: 10.1517/14712598.3.8.1233. Expert Opin Biol Ther. 2003. PMID: 14640949 Review.
-
Herpes vector-mediated gene transfer in treatment of diseases of the nervous system.Annu Rev Microbiol. 2004;58:253-71. doi: 10.1146/annurev.micro.58.030603.123709. Annu Rev Microbiol. 2004. PMID: 15487938 Review.
Cited by
-
Update on the management of diabetic polyneuropathies.Diabetes Metab Syndr Obes. 2011;4:289-305. doi: 10.2147/DMSO.S11324. Epub 2011 Jul 21. Diabetes Metab Syndr Obes. 2011. PMID: 21887102 Free PMC article.
-
Ex vivo nonviral gene delivery of μ-opioid receptor to attenuate cancer-induced pain.Pain. 2017 Feb;158(2):240-251. doi: 10.1097/j.pain.0000000000000750. Pain. 2017. PMID: 28092646 Free PMC article.
-
Prolonged preservation of nerve function in diabetic neuropathy in mice by herpes simplex virus-mediated gene transfer.Diabetologia. 2007 Jul;50(7):1550-8. doi: 10.1007/s00125-007-0702-4. Epub 2007 May 17. Diabetologia. 2007. PMID: 17508196
-
Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons.Brain. 2009 Apr;132(Pt 4):879-88. doi: 10.1093/brain/awp014. Epub 2009 Feb 24. Brain. 2009. PMID: 19244253 Free PMC article.
-
Gene therapy for the treatment of diabetic neuropathy.Curr Diab Rep. 2008 Dec;8(6):431-6. doi: 10.1007/s11892-008-0075-1. Curr Diab Rep. 2008. PMID: 18990298 Free PMC article. Review.
References
-
- Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46 (Suppl 2):S43–S49. - PubMed
-
- Ishii DN. Implication of insulin-like grwoth factors in the pathogenesis of diabetic neuropathy. Brain Res. 1995;20:47–67. - PubMed
-
- Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. - PubMed
-
- Goss JR. Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neruropathy in streptozotocin-induced diabetes in the mouse. Diabetes. 2002;51:2227–2232. - PubMed
-
- Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004;58:253–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

