Practical uses for ecdysteroids in mammals including humans: an update
- PMID: 15844229
- PMCID: PMC524647
- DOI: 10.1093/jis/3.1.7
Practical uses for ecdysteroids in mammals including humans: an update
Abstract
Ecdysteroids are widely used as inducers for gene-switch systems based on insect ecdysteroid receptors and genes of interest placed under the control of ecdysteroid-response elements. We review here these systems, which are currently mainly used in vitro with cultured cells in order to analyse the role of a wide array of genes, but which are expected to represent the basis for future gene therapy strategies. Such developments raise several questions, which are addressed in detail. First, the metabolic fate of ecdysteroids in mammals, including humans, is only poorly known, and the rapid catabolism of ecdysteroids may impede their use as in vivo inducers. A second set of questions arose in fact much earlier with the pioneering "heterophylic" studies of Burdette in the early sixties on the pharmacological effects of ecdysteroids on mammals. These and subsequent studies showed a wide range of effects, most of them being beneficial for the organism (e.g. hypoglycaemic, hypocholesterolaemic, anabolic). These effects are reviewed and critically analysed, and some hypotheses are proposed to explain the putative mechanisms involved. All of these pharmacological effects have led to the development of a wide array of ecdysteroid-containing preparations, which are primarily used for their anabolic and/or "adaptogenic" properties on humans (or horses or dogs). In the same way, increasing numbers of patents have been deposited concerning various beneficial effects of ecdysteroids in many medical or cosmetic domains, which make ecdysteroids very attractive candidates for several practical uses. It may be questioned whether all these pharmacological actions are compatible with the development of ecdysteroid-inducible gene switches for gene therapy, and also if ecdysteroids should be classified among doping substances.
Figures
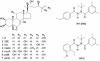


Similar articles
-
The Karlson Lecture. Phytoecdysteroids: what use are they?Arch Insect Biochem Physiol. 2009 Nov;72(3):126-41. doi: 10.1002/arch.20334. Arch Insect Biochem Physiol. 2009. PMID: 19771554 Review.
-
Ecdysteroids: production in plant in vitro cultures.Phytochem Rev. 2017;16(4):603-622. doi: 10.1007/s11101-016-9483-z. Epub 2016 Nov 24. Phytochem Rev. 2017. PMID: 28867986 Free PMC article. Review.
-
The metabolism of 20-hydroxyecdysone in mice: relevance to pharmacological effects and gene switch applications of ecdysteroids.J Steroid Biochem Mol Biol. 2011 Aug;126(1-2):1-9. doi: 10.1016/j.jsbmb.2011.03.016. Epub 2011 Mar 23. J Steroid Biochem Mol Biol. 2011. PMID: 21439380
-
Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals.J Endocrinol. 2006 Oct;191(1):1-8. doi: 10.1677/joe.1.06900. J Endocrinol. 2006. PMID: 17065383 Review.
-
[Ecdysteroids of Silene italica ssp. nemoralis, novel approaches of ecdysteroid therapy].Acta Pharm Hung. 2004;74(3):131-41. Acta Pharm Hung. 2004. PMID: 16318222 Hungarian.
Cited by
-
Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity.Int J Environ Res Public Health. 2020 Sep 4;17(18):6453. doi: 10.3390/ijerph17186453. Int J Environ Res Public Health. 2020. PMID: 32899750 Free PMC article.
-
The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review.Acta Pharm Sin B. 2021 Jul;11(7):1740-1766. doi: 10.1016/j.apsb.2020.10.012. Epub 2020 Oct 16. Acta Pharm Sin B. 2021. PMID: 34386319 Free PMC article. Review.
-
Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.).Compr Rev Food Sci Food Saf. 2015 Jul;14(4):431-445. doi: 10.1111/1541-4337.12135. Epub 2015 Apr 10. Compr Rev Food Sci Food Saf. 2015. PMID: 27453695 Free PMC article.
-
Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities.BMC Complement Altern Med. 2016 Sep 27;16(1):375. doi: 10.1186/s12906-016-1363-y. BMC Complement Altern Med. 2016. PMID: 27677846 Free PMC article.
-
Ecdysteroid hormone action.Cell Mol Life Sci. 2009 Dec;66(24):3837-50. doi: 10.1007/s00018-009-0112-5. Cell Mol Life Sci. 2009. PMID: 19669094 Free PMC article. Review.
References
-
- Abeysinghe RD, Greene BT, Haynes R, Willingham MC, Turner JL, Planalp RP, Brechbeil MW, Torti FM, Torti SV. p53-Independent apoptosis mediated by tachpyridine, an anti-cancer iron chelator. Carcinogenesis. 2001;22:1607–1614. - PubMed
-
- Abubakirov NK, Sultanov MB, Syrov VN, Kurmukov AG, Baltaev U, Novosel'skaya IL, Mamatkhanov AV, Gorovits MB, Shakirov TT, Shamsutdinov I, Yakubova MR, and Genkinoy GL. 1988 Tonic preparation containing the phytoecdysteroid (ecdystene). Application SU 1312774 (Chemical Abstracts 110:121377).
-
- Ahmad VU, Khaliq-Uz-Zaman SM, Ali MS, Perveen S, and Ahmed W. 1996 An antimicrobial ecdysone from Asparagus dumosus. Fitoterapia. LXVII. 1:88–91.
-
- Aikake A, Matsumoto T, and Yamaguchi Y. 1996 Cerebral neuron protective agents containing ecdysteroids. Application JP 94-195279/19940819 (Chemical Abstracts 125:1395).
-
- Aizikov MI, Kurmukov AG, and Syrov VN. 1978 Physiological activity and correlative changes in protein, carbohydrate, and fat metabolism under the effect of ecdysone and nerobol. Farmakologiya Prirodnykh Veschestv. 107–125.(Chemical Abstracts 90 : 180683).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

