Trypanosomes expressing a mosaic variant surface glycoprotein coat escape early detection by the immune system
- PMID: 15845470
- PMCID: PMC1087325
- DOI: 10.1128/IAI.73.5.2690-2697.2005
Trypanosomes expressing a mosaic variant surface glycoprotein coat escape early detection by the immune system
Abstract
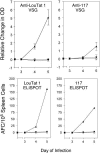
Host resistance to African trypanosomiasis is partially dependent on an early and strong T-independent B-cell response against the variant surface glycoprotein (VSG) coat expressed by trypanosomes. The repetitive array of surface epitopes displayed by a monotypic surface coat, in which identical VSG molecules are closely packed together in a uniform architectural display, cross-links cognate B-cell receptors and initiates T-independent B-cell activation events. However, this repetitive array of identical VSG epitopes is altered during the process of antigenic variation, when former and nascent VSG proteins are transiently expressed together in a mosaic surface coat. Thus, T-independent B-cell recognition of the trypanosome surface coat may be disrupted by the introduction of heterologous VSG molecules into the coat structure. To address this hypothesis, we transformed Trypanosoma brucei rhodesiense LouTat 1 with the 117 VSG gene from Trypanosoma brucei brucei MiTat 1.4 in order to produce VSG double expressers; coexpression of the exogenous 117 gene along with the endogenous LouTat 1 VSG gene resulted in the display of a mosaic VSG coat. Results presented here demonstrate that the host's ability to produce VSG-specific antibodies and activate B cells during early infection with VSG double expressers is compromised relative to that during infection with the parental strain, which displays a monotypic coat. These findings suggest a previously unrecognized mechanism of immune response evasion in which coat-switching trypanosomes fail to directly activate B cells until coat VSG homogeneity is achieved. This process affords an immunological advantage to trypanosomes during the process of antigenic variation.
Figures





Similar articles
-
Variant surface glycoprotein density defines an immune evasion threshold for African trypanosomes undergoing antigenic variation.Nat Commun. 2017 Oct 10;8(1):828. doi: 10.1038/s41467-017-00959-w. Nat Commun. 2017. PMID: 29018220 Free PMC article.
-
Independent regulation of B cell responses to surface and subsurface epitopes of African trypanosome variable surface glycoproteins.J Immunol. 1988 Jul 15;141(2):620-6. J Immunol. 1988. PMID: 2454998
-
T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice.Infect Immun. 1990 Jul;58(7):2337-42. doi: 10.1128/iai.58.7.2337-2342.1990. Infect Immun. 1990. PMID: 1694824 Free PMC article.
-
A Host-Pathogen Interaction Reduced to First Principles: Antigenic Variation in T. brucei.Results Probl Cell Differ. 2015;57:23-46. doi: 10.1007/978-3-319-20819-0_2. Results Probl Cell Differ. 2015. PMID: 26537376 Review.
-
Escaping the immune system by DNA repair and recombination in African trypanosomes.Open Biol. 2019 Nov 29;9(11):190182. doi: 10.1098/rsob.190182. Epub 2019 Nov 13. Open Biol. 2019. PMID: 31718509 Free PMC article. Review.
Cited by
-
Telomere and Subtelomere R-loops and Antigenic Variation in Trypanosomes.J Mol Biol. 2020 Jul 10;432(15):4167-4185. doi: 10.1016/j.jmb.2019.10.025. Epub 2019 Nov 2. J Mol Biol. 2020. PMID: 31682833 Free PMC article. Review.
-
Variant surface glycoprotein density defines an immune evasion threshold for African trypanosomes undergoing antigenic variation.Nat Commun. 2017 Oct 10;8(1):828. doi: 10.1038/s41467-017-00959-w. Nat Commun. 2017. PMID: 29018220 Free PMC article.
-
Parasite-intrinsic factors can explain ordered progression of trypanosome antigenic variation.Proc Natl Acad Sci U S A. 2007 May 8;104(19):8095-100. doi: 10.1073/pnas.0606206104. Epub 2007 Apr 26. Proc Natl Acad Sci U S A. 2007. PMID: 17463092 Free PMC article.
-
Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity.Front Immunol. 2016 Jun 24;7:233. doi: 10.3389/fimmu.2016.00233. eCollection 2016. Front Immunol. 2016. PMID: 27446070 Free PMC article. Review.
-
Maintaining the protective variant surface glycoprotein coat of African trypanosomes.Biochem Soc Trans. 2005 Nov;33(Pt 5):981-2. doi: 10.1042/BST20050981. Biochem Soc Trans. 2005. PMID: 16246026 Free PMC article.
References
-
- Agur, Z., D. Abiri, and L. H. Van der Ploeg. 1989. Ordered appearance of antigenic variants of African trypanosomes explained in a mathematical model based on a stochastic switch process and immune-selection against putative switch intermediates. Proc. Natl. Acad. Sci. USA 86:9626-9630. - PMC - PubMed
-
- Alexander, D. L., K. J. Schwartz, A. E. Balber, and J. D. Bangs. 2002. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 115:3253-3263. - PubMed
-
- Bachmann, M. F., and R. Zinkernagel. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17:553-558. - PubMed
-
- Baltz, T., C. Giroud, D. Baltz, C. Roth, A. Raibaud, and H. Eisen. 1986. Stable expression of two variable surface glycoproteins by cloned Trypanosoma equiperdum. Nature 319:602-604. - PubMed
-
- Bangs, J. D. 1998. Surface coats and secretory trafficking in African trypanosomes. Curr. Opin. Microbiol. 1:448-454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

