Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines
- PMID: 15845475
- PMCID: PMC1087348
- DOI: 10.1128/IAI.73.5.2728-2735.2005
Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines
Abstract
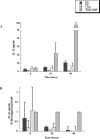
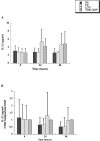
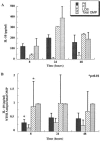
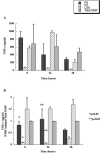
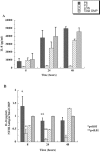
Interactions of nontypeable Haemophilus influenzae (NTHI) with human macrophages contribute to the pathogenesis of NTHI-induced infection in humans. However, the immunologic mechanisms that initiate and perpetuate NTHI-mediated macrophage responses have not been well explored. Outer membrane protein (OMP) P6 is a conserved lipoprotein expressed by NTHI in vivo that possesses a Pam(3)Cys terminal motif, characteristic of immunoactive bacterial lipoproteins associated with Toll-like receptor signaling. We theorized that OMP P6 is a potent immunomodulator of human macrophages. To test this hypothesis, we purified OMP P6 as well as OMP P2, the predominant NTHI outer membrane protein, and lipooligosaccharide (LOS), the specific endotoxin of NTHI, from NTHI strain 1479. Human blood monocyte-derived macrophages, purified from healthy donors, were incubated with each outer membrane constituent, and cytokine production of macrophage supernatants interleukin-1beta (IL-1beta), tumor necrosis factor alpha (TNF-alpha), IL-10, IL-12, and IL-8 was measured. OMP P6 selectively upregulated IL-10, TNF-alpha, and IL-8. While OMP P6 (0.1 mug/ml for 8 h) elicited slightly greater concentrations of IL-10, it resulted in over ninefold greater concentrations of TNF-alpha and over fourfold greater concentrations of IL-8 than did OMP P2. OMP P6 at doses as low as 10 pg/ml was still effective at induction of macrophage IL-8, while OMP P2 and LOS were not. OMP P6 of NTHI is a specific trigger of bacteria-induced human macrophage inflammatory events, with IL-8 and TNF-alpha as key effectors of P6-induced macrophage responses.
Figures


References
-
- Abe, Y., T. F. Murphy, S. Sethi, H. S. Faden, J. Dmochowski, Y. Harabuchi, and Y. M. Thanavala. 2002. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 165:967-971. - PubMed
-
- Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. - PubMed
-
- Badr, W. H., D. Loghmanee, R. J. Karalus, T. F. Murphy, and Y. Thanavala. 1999. Immunization of mice with P6 of nontypeable Haemophilus influenzae: kinetics of the antibody response and IgG subclasses. Vaccine 18:29-37. - PubMed
-
- Berenson, C. S., M. A. Gallery, J. M. Smigiera, and R. H. Rasp. 2002. The role of ceramide of human macrophage gangliosides in activation of human macrophages. J. Leukoc. Biol. 72:492-502. - PubMed
-
- Berenson, C. S., M. A. Patterson, M. A. Pattoli, and T. F. Murphy. 1996. A monoclonal antibody to human macrophage gangliosides inhibits macrophage migration. J. Leukoc. Biol. 59:371-379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

