Induction of strain-transcending immunity against Plasmodium chabaudi adami malaria with a multiepitope DNA vaccine
- PMID: 15845504
- PMCID: PMC1087359
- DOI: 10.1128/IAI.73.5.2974-2985.2005
Induction of strain-transcending immunity against Plasmodium chabaudi adami malaria with a multiepitope DNA vaccine
Abstract
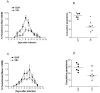
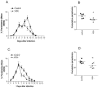
A major goal of current malaria vaccine programs is to develop multivalent vaccines that will protect humans against the many heterologous malaria strains that circulate in endemic areas. We describe a multiepitope DNA vaccine, derived from a genomic Plasmodium chabaudi adami DS DNA expression library of 30,000 plasmids, which induces strain-transcending immunity in mice against challenge with P. c. adami DK. Segregation of this library and DNA sequence analysis identified vaccine subpools encoding open reading frames (ORFs)/peptides of >9 amino acids [aa] (the V9+ pool, 303 plasmids) and >50 aa (V50+ pool, 56 plasmids), respectively. The V9+ and V50+ plasmid vaccine subpools significantly cross-protected mice against heterologous P. c. adami DK challenge, and protection correlated with the induction of both specific gamma interferon production by splenic cells and opsonizing antibodies. Bioinformatic analysis showed that 22 of the V50+ ORFs were polypeptides conserved among three or more Plasmodium spp., 13 of which are predicted hypothetical proteins. Twenty-nine of these ORFs are orthologues of predicted Plasmodium falciparum sequences known to be expressed in the blood stage, suggesting that this vaccine pool encodes multiple blood-stage antigens. The results have implications for malaria vaccine design by providing proof-of-principle that significant strain-transcending immunity can be induced using multiepitope blood-stage DNA vaccines and suggest that both cellular responses and opsonizing antibodies are necessary for optimal protection against P. c. adami.
Figures





Similar articles
-
Induction of specific T-cell responses, opsonizing antibodies, and protection against Plasmodium chabaudi adami infection in mice vaccinated with genomic expression libraries expressed in targeted and secretory DNA vectors.Infect Immun. 2003 Aug;71(8):4506-15. doi: 10.1128/IAI.71.8.4506-4515.2003. Infect Immun. 2003. PMID: 12874330 Free PMC article.
-
Vaccination with a Plasmodium chabaudi adami multivalent DNA vaccine cross-protects A/J mice against challenge with P. c. adami DK and virulent Plasmodium chabaudi chabaudi AS parasites.Int J Parasitol. 2008 Jun;38(7):819-27. doi: 10.1016/j.ijpara.2007.10.009. Epub 2007 Nov 20. Int J Parasitol. 2008. PMID: 18062974
-
A bicistronic DNA vaccine containing apical membrane antigen 1 and merozoite surface protein 4/5 can prime humoral and cellular immune responses and partially protect mice against virulent Plasmodium chabaudi adami DS malaria.Infect Immun. 2004 Oct;72(10):5565-73. doi: 10.1128/IAI.72.10.5565-5573.2004. Infect Immun. 2004. PMID: 15385453 Free PMC article.
-
Immunity to erythrocytic stages of malarial parasites.Am J Trop Med Hyg. 1994;50(4 Suppl):27-32. doi: 10.4269/ajtmh.1994.50.27. Am J Trop Med Hyg. 1994. PMID: 7909653 Review.
-
The immunological challenge to developing a vaccine to the blood stages of malaria parasites.Immunol Rev. 2004 Oct;201:254-67. doi: 10.1111/j.0105-2896.2004.00178.x. Immunol Rev. 2004. PMID: 15361246 Review.
Cited by
-
Synthetic Plasmodium-like hemozoin activates the immune response: a morphology - function study.PLoS One. 2009 Sep 9;4(9):e6957. doi: 10.1371/journal.pone.0006957. PLoS One. 2009. PMID: 19742308 Free PMC article.
-
Safety, immunogenicity, and cross-species protection of a plasmid DNA encoding Plasmodium falciparum SERA5 polypeptide, microbial epitopes and chemokine genes in mice and olive baboons.J Biomed Res. 2017 Jul 13;31(4):321-332. doi: 10.7555/JBR.31.20160025. J Biomed Res. 2017. PMID: 28808204 Free PMC article.
-
Macrophage migration inhibitory factor: a downregulator of early T cell-dependent IFN-gamma responses in Plasmodium chabaudi adami (556 KA)-infected mice.J Immunol. 2011 Jun 1;186(11):6271-9. doi: 10.4049/jimmunol.1003355. Epub 2011 Apr 25. J Immunol. 2011. PMID: 21518974 Free PMC article.
References
-
- Anders, R. F., P. E. Crewthe, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. - PubMed
-
- Barry, M. A., W. C. Lai, and S. A. Johnston. 1995. Protection against mycoplasma infection using expression-library immunization. Nature 377:632-635. - PubMed
-
- Bowman, S., D. Lawson, D. Basham, D. Brown, T. Chillingworth, C. M. Churcher, A. Craig, R. M. Davies, K. Devlin, T. Feltwell, et al. 1999. The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature 400:532-538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

