Cytolethal distending toxin (CDT)-negative Campylobacter jejuni strains and anti-CDT neutralizing antibodies are induced during human infection but not during colonization in chickens
- PMID: 15845513
- PMCID: PMC1087314
- DOI: 10.1128/IAI.73.5.3053-3062.2005
Cytolethal distending toxin (CDT)-negative Campylobacter jejuni strains and anti-CDT neutralizing antibodies are induced during human infection but not during colonization in chickens
Abstract
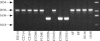
The cytolethal distending toxin (CDT) of Campylobacter jejuni was detectable, using an in vitro assay, in most but not all of 24 strains tested. The reason for the absence of toxin activity in these naturally occurring CDT-negative C. jejuni strains was then investigated at the genetic level. CDT is encoded by three highly conserved genes, cdtA, -B, and -C. In the CDT-negative strains, two types of mutation were identified. The CDT activities of C. jejuni strains possessing both types of mutation were successfully complemented with the functional genes of C. jejuni 11168. The first type of mutation comprised a 667-bp deletion across cdtA and cdtB and considerable degeneration in the remainder of the cdt locus. Using a PCR technique to screen for this deletion, this mutation occurred in fewer than 3% of 147 human, veterinary, and environmental strains tested. The second type of mutation involved at least four nonsynonymous nucleotide changes, but only the replacement of proline with serine at CdtB position 95 was considered important for CDT activity. This was confirmed by site-directed mutagenesis. This type of mutation also occurred in fewer than 3% of strains as determined using a LightCycler biprobe assay. The detection of two CDT-negative clinical isolates raised questions about the role of CDT in some cases of human campylobacteriosis. To determine if anti-CDT antibodies are produced in human infection, a toxin neutralization assay was developed and validated using rabbit antisera. Pooled human sera from infected patients neutralized the toxin, indicating expression and immunogenicity during infection. However, no neutralizing antibodies were detected in colonized chickens despite the expression of CDT in the avian gut as indicated by reverse transcription-PCR.
Figures


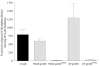

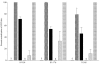
 ), strain EF (R43) (▪), pooled campylobacteriosis patient sera (
), strain EF (R43) (▪), pooled campylobacteriosis patient sera ( ), or pooled experimentally colonized chicken sera (∥) prior to CDT activity assays. Sera from pooled normal human blood donors (▧) as well as sera from preimmunized rabbits (§) and uncolonized chickens (data not shown) were used as controls. The lysates were tested for CDT activity and the percent neutralization determined by comparison with untreated lysates. Error bars indicate standard deviations.
), or pooled experimentally colonized chicken sera (∥) prior to CDT activity assays. Sera from pooled normal human blood donors (▧) as well as sera from preimmunized rabbits (§) and uncolonized chickens (data not shown) were used as controls. The lysates were tested for CDT activity and the percent neutralization determined by comparison with untreated lysates. Error bars indicate standard deviations.
References
-
- Ausubel, S. F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
-
- Bang, D. D., F. Scheutz, P. Ahrens, K. Pedersen, J. Blom, and M. Madsen. 2001. Prevalence of cytolethal distending toxin (cdt) genes and CDT production in Campylobacter spp. isolated from Danish broilers. J. Med. Microbiol. 50:1087-1094. - PubMed
-
- Cawthraw, S., R. Ayling, P. Nuijten, T. Wassenaar, and D. G. Newell. 1994. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 38:341-349. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

