A novel class of dual-family immunophilins
- PMID: 15845546
- PMCID: PMC2270415
- DOI: 10.1074/jbc.M500990200
A novel class of dual-family immunophilins
Abstract
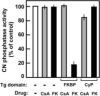
Immunophilins are protein chaperones with peptidylprolyl isomerase activity that belong to one of two large families, the cyclosporin-binding cyclophilins (CyPs) and the FK506-binding proteins (FKBPs). Each family displays characteristic and conserved sequence features that differ between the two families. We report a novel group of dual-family immunophilins that contain both CyP and FKBP domains for which we propose the name FCBP (FK506- and cyclosporin-binding protein). The FCBP of Toxoplasma gondii, a protozoan parasite, contained N-terminal FKBP and C-terminal CyP domains joined by tetratricopeptide repeats. Structure-function analysis revealed that both domains were functional and exhibited family-specific drug sensitivity. The individual domains of FCBP inhibited calcineurin (protein phosphatase 2B) in the presence of the appropriate drugs. In binding studies, FCBP recruited calcineurin in the presence of FK506 and a putative target of rapamycin homolog in the presence of rapamycin. Two additional FCBP sequences in Flavobacterium and one in Treponema (spirochete) were also identified in which the CyP and FKBP domains were in the reverse order. T. gondii growth was inhibited by cyclosporin and FK506 in a moderately synergistic manner. The knockdown of FCBP by RNA interference revealed its essentiality for T. gondii growth. Clearly, the FCBPs are novel chaperones and potential targets of multiple immunosuppressant drugs.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

