The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation
- PMID: 15845764
- PMCID: PMC1088372
- DOI: 10.1073/pnas.0500792102
The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation
Abstract
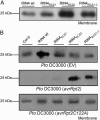
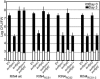
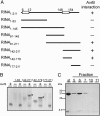
Plant pathogenic Pseudomonas syringae deliver type III effector proteins into the host cell, where they function to manipulate host defense and metabolism to benefit the extracellular bacterial colony. The activity of these virulence factors can be monitored by plant disease resistance proteins deployed to "guard" the targeted host proteins. The Arabidopsis RIN4 protein is targeted by three different type III effectors. Specific manipulation of RIN4 by each of them leads to activation of either the RPM1 or RPS2 disease resistance proteins. The type III effector AvrRpt2 is a cysteine protease that is autoprocessed inside the host cell where it activates RPS2 by causing RIN4 disappearance. RIN4 contains two sites related to the AvrRpt2 cleavage site (RCS1 and RCS2). We demonstrate that AvrRpt2-dependent cleavage of RIN4 at RCS2 is functionally critical in vivo. This event leads to proteasome-mediated elimination of all but a membrane-embedded approximately 6.4-kDa C-terminal fragment of RIN4. One or more of three consecutive cysteines in this C-terminal fragment are required for RIN4 localization; these are likely to be palmitoylation and/or prenylation sites. AvrRpt2-dependent cleavage at RCS2, and release of the remainder of RIN4 from the membrane, consequently prevents RPM1 activation by AvrRpm1 or AvrB. RCS2 is contained within the smallest tested fragment of RIN4 that binds AvrB in vitro. Thus, at least two bacterial virulence factors target the same domain of RIN4, a approximately 30-aa plant-specific signature sequence found in a small Arabidopsis protein family that may be additional targets for these bacterial virulence factors.
Figures



Similar articles
-
Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1.Plant Cell. 2004 Oct;16(10):2822-35. doi: 10.1105/tpc.104.024117. Epub 2004 Sep 10. Plant Cell. 2004. PMID: 15361584 Free PMC article.
-
RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis.Cell. 2002 Mar 22;108(6):743-54. doi: 10.1016/s0092-8674(02)00661-x. Cell. 2002. PMID: 11955429
-
Membrane release and destabilization of Arabidopsis RIN4 following cleavage by Pseudomonas syringae AvrRpt2.Mol Plant Microbe Interact. 2005 Dec;18(12):1258-68. doi: 10.1094/MPMI-18-1258. Mol Plant Microbe Interact. 2005. PMID: 16478045
-
Plant defense: one post, multiple guards?!Mol Cell. 2003 Feb;11(2):284-6. doi: 10.1016/s1097-2765(03)00072-8. Mol Cell. 2003. PMID: 12620215 Review.
-
Role of RIN4 in Regulating PAMP-Triggered Immunity and Effector-Triggered Immunity: Current Status and Future Perspectives.Mol Cells. 2019 Jul 31;42(7):503-511. doi: 10.14348/molcells.2019.2433. Mol Cells. 2019. PMID: 31362467 Free PMC article. Review.
Cited by
-
Quantitative Interactor Screening with next-generation Sequencing (QIS-Seq) identifies Arabidopsis thaliana MLO2 as a target of the Pseudomonas syringae type III effector HopZ2.BMC Genomics. 2012 Jan 9;13:8. doi: 10.1186/1471-2164-13-8. BMC Genomics. 2012. PMID: 22230763 Free PMC article.
-
Plant NBS-LRR proteins in pathogen sensing and host defense.Nat Immunol. 2006 Dec;7(12):1243-9. doi: 10.1038/ni1410. Nat Immunol. 2006. PMID: 17110940 Free PMC article. Review.
-
Host manipulation by bacterial type III and type IV secretion system effector proteases.Cell Microbiol. 2021 Nov;23(11):e13384. doi: 10.1111/cmi.13384. Epub 2021 Aug 30. Cell Microbiol. 2021. PMID: 34392594 Free PMC article. Review.
-
A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity.Nature. 2007 Jul 19;448(7151):370-4. doi: 10.1038/nature05966. Nature. 2007. PMID: 17637671 Free PMC article.
-
Development of a Rapid in planta BioID System as a Probe for Plasma Membrane-Associated Immunity Proteins.Front Plant Sci. 2018 Dec 18;9:1882. doi: 10.3389/fpls.2018.01882. eCollection 2018. Front Plant Sci. 2018. PMID: 30619431 Free PMC article.
References
-
- Dangl, J. L. & Jones, J. D. G. (2001) Nature 411, 826-833. - PubMed
-
- Alfano, J. R. & Collmer, A. (2004) Annu. Rev. Phytopathol. 42, 385-414. - PubMed
-
- van der Biezen, E. A. & Jones, J. D. G. (1998) Trends Biochem. Sci. 23, 454-456. - PubMed
-
- Van der Hoorn, R. A., De Wit, P. J. & Joosten, M. H. (2002) Trends Plant Sci. 7, 67-71. - PubMed
-
- Grant, M. R., Godiard, L., Straube, E., Ashfield, T., Lewald, J., Sattler, A., Innes, R. W. & Dangl, J. L. (1995) Science 269, 843-846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

