Glucosamine therapy for treating osteoarthritis
- PMID: 15846645
- PMCID: PMC8459392
- DOI: 10.1002/14651858.CD002946.pub2
Glucosamine therapy for treating osteoarthritis
Abstract
Background: Osteoarthritis (OA) is the most common form of arthritis, and it is often associated with significant disability and an impaired quality of life.
Objectives: To review all randomized controlled trials (RCTs) evaluating the effectiveness and toxicity of glucosamine in OA.
Search strategy: We searched MEDLINE, PREMEDLINE, EMBASE, AMED, ACP Journal Club, DARE, CDSR, and the CCTR. We also wrote letters to content experts, and hand searched reference lists of identified RCTs and pertinent review articles. All searches were updated in January 2005.
Selection criteria: Relevant studies met the following criteria: 1) RCTs evaluating the effectiveness and safety of glucosamine in OA, 2) Both placebo controlled and comparative studies were eligible, 3) Both single blinded and double blinded studies were eligible.
Data collection and analysis: Data abstraction was performed independently by two investigators and the results were compared for degree of agreement. Gotzsche's method and a validated tool (Jadad 1996) were used to score the quality of the RCTs. Continuous outcome measures were pooled using standardized mean differences (SMD) as the measure of effect size. Dichotomous outcome measures were pooled using relative risk ratios (RR).
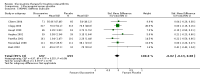
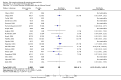
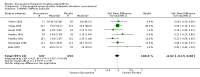
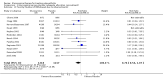
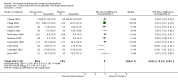
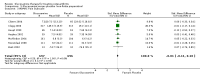
Main results: Analysis restricted to eight studies with adequate allocation concealment failed to show benefit of glucosamine for pain and WOMAC function. Collectively, the 20 analyzed RCTs found glucosamine favoured placebo with a 28% (change from baseline) improvement in pain (SMD -0.61, 95% CI -0.95, -0.28) and a 21% (change from baseline) improvement in function using the Lequesne index (SMD -0.51 95% CI -0.96, -0.05). However, the results are not uniformly positive, and the reasons for this remain unexplained. WOMAC pain, function and stiffness outcomes did not reach statistical significance. In the 10 RCTs in which the Rotta preparation of glucosamine was compared to placebo, glucosamine was found to be superior for pain (SMD -1.31, 95% CI -1.99, -0.64) and function using the Lequesne index (SMD -0.51, 95% CI -0.96, -0.05). Pooled results for pain (SMD -0.15, 95% CI -0.35, 0.05) and function using the WOMAC index (SMD 0.03, 95% CI -0.18, 0.25) in those RCTs in which a non-Rotta preparation of glucosamine was compared to placebo did not reach statistical significance. In the four RCTs in which the Rotta preparation of glucosamine was compared to an NSAID, glucosamine was superior in two, and equivalent in two. Two RCTs using the Rotta preparation showed that glucosamine was able to slow radiological progression of OA of the knee over a three year period (SMD 0.24, 95% CI 0.04, 0.43). Glucosamine was as safe as placebo in terms of the number of subjects reporting adverse reactions (RR=0.97, 95% CI, 0.88, 1.08).
Authors' conclusions: This update includes 20 studies with 2570 patients. Pooled results from studies using a non-Rotta preparation or adequate allocation concealment failed to show benefit in pain and WOMAC function while those studies evaluating the Rotta preparation show that glucosamine was superior to placebo in the treatment of pain and functional impairment resulting from symptomatic OA. WOMAC outcomes of pain, stiffness and function did not show a superiority of glucosamine over placebo for both Rotta and non-Rotta preparations of glucosamine. Glucosamine was as safe as placebo.
Conflict of interest statement
The current version of the 'Glucosamine for osteoarthritis' review on
Dr Tassos Anastassiades submitted patents (Pub. No. US 2002/0045597 A1 and Pub. No. US 6,479,469 B2) for glucosamine type substances in August 2000. The patent protection is for chemically synthesized compounds with substitutions of chain length larger than the naturally occurring acetyl group in glucosamine.
Dr Tanveer Towheed was an invited speaker at the November 2002 ACR meeting. The accredited event, a post‐meeting symposium, was coordinated by the CME department at the Case Western Reserve School of Medicine. Rotta Pharmaceuticals (whose product is glucosamine sulfate) provided an unrestricted educational grant in support of the program. Dr Towheed presented a review of all available published meta‐analyses of various pharmacological therapies for OA. He received a one time honorarium for speaking at this symposium. There is no other association with Rotta Pharmaceuticals, or any other potential conflict of interest.
JB Houpt received an unrestricted grant‐in‐aid of research from Wampole Canada Inc for completion of a trial published in 1999. No industry or other support has been received since.
Figures




































Update of
-
Glucosamine therapy for treating osteoarthritis.Cochrane Database Syst Rev. 2001;(1):CD002946. doi: 10.1002/14651858.CD002946. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Apr 18;(2):CD002946. doi: 10.1002/14651858.CD002946.pub2. PMID: 11279782 Updated.
References
References to studies included in this review
Cibere 2004 {published data only}
-
- Cibere J, Kopec JA, Thorne A, Singer J, Canvin J, Robinson DB, et al. Randomized, double‐blind, placebo‐controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis and Rheumatism (Arthritis Care and Research) 2004;51(2):738‐45. - PubMed
Clegg 2006 {published data only}
-
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. New England Journal of Medicine 2006;354:795‐808. - PubMed
Crolle 1980 {published data only}
-
- Crolle G, D'Este E. Glucosamine sulphate for the management of arthrosis: a controlled clinical investigation. Current Medical Research & Opinion 1980;2:104‐9. - PubMed
D'ambrosio 1981 {published data only}
-
- D'Ambrosio E, Casa B, Bompani R, Scali G, Sclai M. Glucosamine sulphate: a controlled clinical investigation in arthrosis. Pharmatherapeutica 1981;2(8):504‐8. - PubMed
Drovanti 1980 {published data only}
-
- Drovanti A, Bignamini AA, Rovati L. Therapeutic activity of oral glucosamine sulfate in osteoarthrosis: A placebo‐controlled double‐blind investigation. Clinical Therapeutics 1980;3(4):260‐72. - PubMed
Herrero‐Beaumont 2007 {published data only}
-
- Herrero‐Beaumont G, Ivorra JAR, Trabado M, Blanco FJ, Benito P, Martin‐Mola E, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms. Arthritis and Rheumatism 2007;56(2):555‐67. - PubMed
Houpt 1999 {published data only}
-
- Houpt J, McMillan R, Wein C, Paget‐Dellio SD. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. Journal of Rheumatology 1999;26(11):2423‐2430. - PubMed
Hughes 2002 {published data only}
-
- Hughes R, Carr A. A randomized, double‐blind, placebo‐controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology 2002;41:279‐84. - PubMed
McAlindon 2004 {published data only}
-
- McAlindon T, Formica M, LaValley M, Lehmer M, Kabbara K. Effectiveness of Glucosamine for symptoms of knee osteoarthritis: Results from an Internet‐based randomized double‐blind controlled trial. American Journal of Medicine 2004;117:643‐49. - PubMed
Mehta 2007 {published data only}
Muller‐FassBender 94 {published data only}
-
- Muller‐Fasbender H, Bach GL, Haase W, Rovati L, Setnikar I. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthritis and Cartilage 1994;2:61‐9. - PubMed
Noack 1994 {published data only}
-
- Noack W, Fischer M, Forster K, Rovati L, Setnikar I. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthritis and Cartilage 1994;2:51‐9. - PubMed
Pavelka 2002 {published data only}
-
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis. A 3‐year, randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 2002;162:2113‐23. - PubMed
Pujalte 1980 {published data only}
-
- Pujalte JM, Llavore EP, Ylescupidez FR. Double‐blind clinical trial evaluation of oral glucosamine sulphate in the basic treatment of osteoarthrosis. Current Medical Research and Opinion 1980;7(2):110‐14. - PubMed
Qiu 1998 {published data only}
-
- Qiu GX, Gao SN, Giacovelli G, Rovati L, Setnikar I. Efficacy and safety of glucosamine sulfate versus ibuprofen in patients with knee osteoarthritis. Arzneimittelforschung/Drug Resistance Updates 1998;48(1):469‐74. - PubMed
Qiu 2005 {published data only}
-
- Qiu G, Weng X, Zhang K, Zhou Y, Wang LS, Li W, et al. A multicentral, randomized, controlled clinical trial of glucosamine hydrochloride/sulfate in the treatment of knee osteoarthritis. National Medical Journal of China 2005;85(43):3067‐70. - PubMed
Reginster 2001 {published data only}
-
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long‐term effects of glucosamine sulphate on osteoarthritis progression: a randomized, placebo‐controlled clinical trial. Lancet 2001;357:251‐6. - PubMed
Reichelt 1994 {published data only}
-
- Reichelt A, Forster KK, Fischer M, Rovati L, Setnikar I. Efficacy and safety of intramuscular glucosamine sulfate in osteoarthritis of the knee. Arzneimmittelforschung/Drug Resistance Updates 1994;44(1):75‐80. - PubMed
Rindone 2000 {published data only}
Rovati 1997 {published and unpublished data}
-
- Rovati LC. The clinical profile of glucosamine sulfate as a selective symptom‐modifying drug in osteoarthritis: current data and perspectives. Osteoarthritis and Cartilage 1997;5:72.
Rozendaal 2008 {published data only}
-
- Rozendaal RM, Koes BW, m van Osch G, Uitterlinden EJ, Garling EH, Willemsen SP, et al. Effect of glucosamine sulfate on hip osteoarthritis. Annals of Internal Medicine 2008;148:268‐77. - PubMed
Usha 2004 {published data only}
-
- Usha PR, Naidu MUR. Randomized, double‐blind, parallel, placebo‐controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clinical Drug Investigation 2004;24(6):353‐63. - PubMed
Vajaradul 1981 {published data only}
-
- Vajaradul Y. Double‐blind clinical evaluation of intra‐articular glucosamine in outpatients with gonarthrosis. Clinical Therapeutics 1981;3(5):336‐43. - PubMed
Vaz 1982 {published data only}
-
- Vaz AL. Double‐blind clinical evaluation of the relative efficacy of ibuprofen and glucosamine sulphate in the management of osteoarthrosis of the knee in out‐patients. Current Medical Research and Opinion 1982;8:145‐9. - PubMed
Zenk 2002 {published data only}
-
- Zenk JL, Helmer TR, Kuskowski MA. The effects of milk protein concentrate on the symptoms of osteoarthritis in adults: an exploratory, randomized, double‐blind, placebo‐controlled trial. Current Therapeutic Research 2002;63(7):430‐42.
References to studies excluded from this review
Braham 2003 {published data only}
Magi 1997 {unpublished data only}
-
- Magi D, Giacovelli G, Rovati LC. Clinical Study on the activity of glucosamine sulfate on osteoarthritis, in comparison with placebo, in patients suffering from osteoarthritis of the knee by means of imaging techniques (NMR, ecotomography, radiology) and computer processing of the such obtained images. Unpublished technical report prepared by Rottapharm 1997.
Pipitone 1997 {unpublished data only}
-
- Pipitone V, Giacovelli G, Rovati LC. Clinical study on the activity of glucosamine sulfate on the evolution of osteoarthritis in patients with osteoarthritis of the knee. Unpublished technical report prepared by Rottapharm 1997.
Rovati 1993 {unpublished data only}
-
- Rovati LC, Poma A, Biavati G, et al. A multicenter, randomized, double‐blind, parallel‐group study to investigate efficacy and safety of oral glucosamine sulfate in osteoarthritis of the spine. Unpublished technical report prepared by Rottapharm 1993.
Additional references
ACR 2000
-
- American College of Rheumatology (ACR) Subcommittee on osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis and Rheumatism 2000;43:1905‐15. - PubMed
Adebowale 2000
-
- Adebowale AO, Cox DS, Liang Z, Eddington ND. Analysis of glucosamine and chondroitin sulfate content in marketed products and the caco‐2 permeability of chondroitin sulfate raw materials. Journal of the American Nutraceutical Association 2000;3(1):Spring Issue.
Anderson 2005
-
- Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food and Chemical Toxicology 2005;43:187‐201. - PubMed
Audimoolam 2006
-
- Audimoolam VK, Bhandari S. Acute interstitial nephritis induced by glucosamine. Nephrology Dialysis Transplantation 2006;21(7):2031. - PubMed
Badley 1995
-
- Badley EM. The effect of osteoarthritis on disability and health care use in Canada. Journal of Rheumatology 1995;22 Suppl 43:19‐22. - PubMed
Barclay 1998
-
- Barclay TS, Tsourounis C, McCart GM. Glucosamine. Annals of Pharmacotherapy 1998;32:574‐9. - PubMed
Bassleer 1992
-
- Bassleer C, Henrotin Y, Franchimont P. In‐vitro evaluation of drugs proposed as chondroprotective agents. International Journal of Tissue Reactions 1992;14:231‐41. - PubMed
Bassleer 1998
-
- Bassleer C, Rovati L, Franchimont P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic cartilage in vitro. Osteoarthritis and Cartilage 1998;6:427‐34. - PubMed
Bellamy 1997
-
- Bellamy N, Kirwan J, Boers M, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip and hand osteoarthritis. Journal of Rheumatology 1997;24:799‐802. - PubMed
Biggee 2006
Biggee 2007
Chan 2001
-
- Chan NN, Baldeweg S, Tan TM, Hurel S. Glucosamine sulphate and osteoarthritis (letter). Lancet 2001;357:1617. - PubMed
Cohen 1977
-
- Cohen J. Statistical power analysis for the behavioural sciences. Statistical power analysis for the behavioural sciences. New York: Academic Press, 1977.
Conrozier 1998
-
- Conrozier T, Mathieu P, Piperno M, et al. Glucosamine sulfate significantly reduced cartilage destruction in a rabbit model os osteoarthritis. Arthritis and Rheumatism 1998;41 Suppl:147.
Consumer Reports 2002
-
- No authors listed. Joint Remedies. Consumer Reports 2002;67(1):18‐21. - PubMed
Creamer 1997
-
- Creamer P, Hochberg MC. Osteoarthritis. Lancet 1997;350:503‐8. - PubMed
Creamer 1998
-
- Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clinics in Geriatric Medicine 1998;14(3):434‐54. - PubMed
Da Camara 1998
-
- Camara C, Dowless GV. Glucosamine sulfate for osteoarthritis. Annals of Pharmacotherapy 1998;32:580‐7. - PubMed
Deal 1999
-
- Deal CL, Moskowitz RW. Neutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chrondroitin sulfate and collagen hydrolysate. Rheumatic Diseases Clinics of North America 1999;25:379‐95. - PubMed
Deviere 2002
-
- Deviere J. Do selective cyclo‐oxygenase inhibitors eliminate the adverse events associated with nonsteroidal anti‐inflammatory drug therapy?. European Journal of Gastroenterology and Hepatology 2002;14 Suppl 1:29‐33. - PubMed
Felson 1998
-
- Felson D. Nonmedicinal therapies for osteoarthritis. Bulletin on the Rheumatic Diseases 1998;47(2):1‐7. - PubMed
Felson 2000
-
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG. Osteoarthritis: new insights. Part 1: the disease and its prevalence and impact. Annals of Internal Medicine 2000;133(8):635‐46. - PubMed
Gabriel 1991
-
- Gabriel S, Jaakkimainen L, Bombardier C. Risk factors for serious gastrointestinal complications related to use of non‐steroidal anti‐inflammatory drugs. A meta‐analysis. Annals of Internal Medicine 1991;115:787‐96. - PubMed
Garner 2002
Goldstein 2001
-
- Goldstein MR. Glucosamine sulphate and osteoarthritis (letter). Lancet 2001;357:1617‐8. - PubMed
Griffin 1991
-
- Griffin M, Piper J, Daugherty J, et al. Non‐steroidal anti‐inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Annals of Internal Medicine 1991;114:257‐63. - PubMed
Hedges 1985
-
- Hedges L, Olkin I. Statistical methods for meta‐analysis. San Diego, CA: Academic Press, 1995.
Herman 1986
-
- Herman J, Appel A, Khosla R, et al. The in vitro effect of select classes of non‐steroidal anti‐inflammatory drugs on normal cartilage metabolism. Journal of Rheumatology 1986;13:1014‐8. - PubMed
Hochberg 1995a
-
- Hochberg MC, Altman R, Brandt K, et al. Guidelines for the medical management of osteoarthritis. Part 1. Osteoarthritis of the hip. Arthritis and Rheumatism 1995;38(11):1535‐40. - PubMed
Hochberg 1995b
-
- Hochberg MC, Altman R, Brandt K, et al. Guidelines for the medical management of osteoarthritis. Part 2. Osteoarthritis of the knee. Arthritis and Rheumatism 1995;38(11):1541‐7. - PubMed
Hochberg 1996
-
- Hochberg MC, Perlmutter D, Hudson J, et al. Preferences in the management of osteoarthritis of the hip and knee: Results of a survey of community‐based rheumatologists in the United States. Arthritis Care and Research 1996;9(3):170‐6. - PubMed
Hoffer 2002
-
- Hoffer LJ, Kaplan LN, Hamadeh MJ, Grigoriu AC, Baron M. Sulfate could mediate the therapeutic effect of glucosamine sulfate. Metabolism 2002;50(7):767‐70. - PubMed
Jadad 1996
-
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of reports of randomized trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Largo 2003
-
- Largo R, Alvarez‐Soria MA, Diez‐Ortego I, et al. Glucosamine inhibits IL‐1 beta induced NFKB activation in human osteoarthritic chondrocytes. Osteoarthritis and Cartilage 2003;11:290‐8. - PubMed
Laverty 2005
-
- Laverty S, Sandy JD, Celeste C, Vachon P, Marier JF, Plaas AHK. Synovial fluid levels and serum pharmacokinetics in a large animal model following treatment with oral glucosamine at clinically relevant doses. Arthritis and Rheumatism 2005;52:181‐91. - PubMed
Lawrence 1998
-
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis and Rheumatism 1998;41(5):778‐99. - PubMed
Liang 1984
-
- Liang M, Larsen M, Thomson M, Eaton H, Mcnamara E, Katz R, et al. Costs and outcoes in rheumatoid arthritis and osteoarthritis. Arthritis and Rheumatism 1984;27(5):522‐9. - PubMed
Lozada 1997
-
- Lozada C, Altman R. Chrondroprotection in osteoarthritis. Bulletin on the Rheumatic Diseases 1997;46:5‐7. - PubMed
Matheu 1999
-
- Matheu V, Gracia Bara MT, Pelta R, Vivas E, Rubio M. Immediate‐hypersensitivity reaction to glucosamine sulfate. Allergy 1999;54:643‐50. - PubMed
McAlindon 2000
-
- McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and Chondroitin for Treatment of Osteoarthritis. JAMA 2000;283(11):1469‐1475. - PubMed
McAlindon 2003
-
- McAlindon T. Why are clinical trials of glucosamine no longer uniformly positive?. Rheumatic Diseases Clinics of North America 2003;29(4):789‐801. - PubMed
McCarty 1994
-
- McCarty M. The neglect of gulcosamine as a treatment for osteoarthritis. A personal perspective. Medical Hypotheses 1994;42:323‐327. - PubMed
Monauni 2000
-
- Monauni T, Zenti MG, Cretti A, Daniels MC, Targher G, Caruso B, et al. Effects of glucosamine infusion on insulin secretion and insulin action on humans. Diabetes 2000;49(6):926‐35. - PubMed
Moralestorres 1996
-
- Moralestorres J, Reginster J, Hochberg MC. Rheumatic and musculoskeletal diseases and impared quality of life. A challenge for rheumatologists. Journal of Rheumatology 1996;23(1):1‐3. - PubMed
Mroz 2004
-
- Mroz PJ, Silbert JE. Use of 3H‐glucosamine and 35S‐sulfate with cultured human chondrocytes to determine the effect of glucosamine concentration on formation of chondroitin sulfate. Arthritis and Rheumatism 2004;50:3574‐9. - PubMed
Muniyappa 2006
-
- Muniyappa R, Karne RJ, Hall G, Crandon SK, Bronstein JA, Ver MR, et al. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes 2006;55(11):3142‐50. - PubMed
Persiani 2005a
-
- Persiani S, Roda E, Rovati LC, Locatelli M, Giacovelli G, Roda A. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthritis and Cartilage 2005;13:1041‐9. - PubMed
Persiani 2005b
-
- Persiani S, Rotini R, Rovati LC, Locatelli M, Roda A. Glucosamine plasma and synovial fluid concentrations before and oral administration of crystalline glucosamine sulfate in man. Annals of the Rheumatic Diseases. 2005; Vol. 64, issue Suppl III:490.
Petitti 1994
-
- Petitti D. Meta‐analysis, Decision analysis, and Cost‐Effectiveness analysis. Monographs in Epidemiology and Biostatistics. Vol. 24, Oxford University Press, 1994.
Pham 2007
-
- Pham T, Cornea A, Blick KE, Jenkins A, Scofield RH. Oral glucosamine in doses used to treat osteoarthritis worsens insulin resistance. American Journal of the Medical Sciences 2007;333(6):333‐9. - PubMed
Puett 1994
-
- Puett D, Griffin M. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Annals of Internal Medicine 1994;121:133‐40. - PubMed
Rashad 1989
-
- Rashad S, Revell P, Hemingway A, Low F, Rainsford K, Walker F. Effect of non‐steroidal anti‐inflammatory drugs on the course of osteoarthritis. Lancet 1989;2:519‐22. - PubMed
Reginister 2003
-
- Reginister J, Bruyere O, Lecart M, Henroitin Y. Naturocetic (glucosamine and chondroitin sulfate) compounds as structure‐modifying drugs in the treatment of osteoarthritis. Current Opinion in Rheumatology 2003;15(5):651‐5. - PubMed
Richy 2003
-
- Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin, Reginster JY. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis. A comprehensive meta‐analysis. Archives of Internal Medicine 2003;163:1514‐22. - PubMed
Roden 1956
-
- Roden L. Effect of hexosamines on the synthesis of chrondroitin sulphruic acid in vitro. Arkhiv Foer Kemi 1956;10:345‐352.
Russell 2002
-
- Russell AS, Aghazadeh‐Habashi A, Jamali F. Active ingredient consistency of commercially available glucosamine sulfate products. Journal of Rheumatology 2002;29(11):2407‐9. - PubMed
Schulz 1995a
-
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Scott 1993
-
- Scott JC, Hochberg MC. Arthritic and other musculoskeletal diseases. In: Brownson RC, Remington PL, Davis JR editor(s). Chronic Disease Epidemiology and Control. Washington: American Public Health Association, 1993.
Scroggie 2003
-
- Scroggie DA, Albright A, Harris MD. The effect of glucosamine‐chondroitin supplementation on glycosylated hemaglobin levels in patients with type 2 diabetes mellitus: a placebo‐controlled, double‐blinded, randomized controlled trial. Archives of Internal Medicine 2003;163(13):1587‐90. - PubMed
Setnikar 1984
-
- Setnikar I, Giachetti C, Zanolo G. Absorption, distribution and excretion of radioactivity after a single intravenous or oral administration of 14C‐glucosamine to the rat. Pharmatherapeutica 1984;3:523‐30. - PubMed
Setnikar 1986
-
- Setnikar I, Giachetti C, Zanolo G. Pharmacokinetics of glucosamine in dog and man. Arzneimittelforschung 1986;36:729‐35. - PubMed
Setnikar 1991a
-
- Setnikar I, Pacini M, Revel L. Antiarthritic effects of glucosamine sulfate studied in animal mdoels. Arzneimittelforschung 1991;41:542‐5. - PubMed
Setnikar 1991b
-
- Setnikar I, Cereda R, pacini MA, Revel L. Antireactive properties of glucosamine sulfate. Arneimittelforschung 1991;41:157‐61. - PubMed
Setnikar 1992
-
- Setnikar I. Antireactive properties of chondroprotective drugs. International Journal of Tissue Reactions 1992;24:253‐61. - PubMed
Setnikar 1993
-
- Setnikar I, Palumbo R, Canali S, Zanolo G. Pharmacokinetics of glucosamine in man. Arzneimittelforschung 1993;43:1109‐13. - PubMed
Setnikar 2001
-
- Setnikar I, Rovati LC. Absorption, distribution, metabolism and excretion of glucosamine sulfate. Arzneimittelforschung/Drug Resistance Updates 2001;51(2):699‐725. - PubMed
Shikhman 2001
-
- Shikhman AR, Kuhn K, Alaaeddine N, Lotz M. N‐acetylglucosamine prevents IL‐1 beta‐mediated activation of human chondrocytes. Journal of Immunology 2001;166:5155‐60. - PubMed
Sibbald 2004
Sivojelezova 2007
-
- Sivojelezova A, Koren G, Einarson A. Glucosamine use in pregnancy: an evaluation of pregnancy outcome. Journal of Women's Health 2007;16(3):345‐8. - PubMed
Swinburne 2001
-
- Swinburne LM. Glucosamine sulphate and osteoarthritis (letter). Lancet 2001;357:1617. - PubMed
Tallia 2002
-
- Tallia AF, Cardone DA. Asthma exacerbation associated with glucosamine‐chondroitin supplement. The Journal of the American Board of Family Practice 2002;15:481‐4. - PubMed
Tannis 2004
-
- Tannis AJ, Barban J, Conquer JA. Effect of glucosamine supplementation on fasting and non‐fasting plasma glucose and serum insulin concentration in healthy individuals. Osteoarthritis and Cartilage 2004;12(6):506‐11. - PubMed
Tapadinhas 1982
-
- Tapadinhas MJ, Rivera IC, Bignamini AA. Oral glucosamine sulphate in the management of arthrosi: report on a mult‐centre open investigation in Portugal. Pharmacotherapeutica 1982;3:157‐68. - PubMed
Towheed 1997a
-
- Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the hip. Journal of Rheumatology 1997;24:549‐57. - PubMed
Towheed 1997b
-
- Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Seminars in Arthritis and Rheumatism 1997;26:755‐70. - PubMed
Towheed 1998
-
- Towheed TE. Impact of musculoskeletal disorders in Canada. Annals of the Royal College of Physicians and Surgeons of Canada 1998;31(5):229‐32.
Towheed 1999
-
- Towheed TE, Anatassiades TP. Glucosamine therapy for osteoarthritis (editorial). Journal of Rheumatology 1999;26(11):2294‐7. - PubMed
Towheed 2000
-
- Towheed TE. Glucosamine and chondroitin for treating symptoms of osteoarthritis: evidence is widely touted but incomplete. JAMA 2000;283(11):1483‐4. - PubMed
Towheed 2002
-
- Towheed TE. Published meta‐analyses of pharmacological therapies for osteoarthritis. Osteoarthritis and Cartilage 2002;10(11):836‐7. - PubMed
Towheed 2003
-
- Towheed TE. Current Status of glucosamine therapy in osteoarthritis. Arthritis and Rheumatism 2003;49(4):601‐4. - PubMed
Towheed 2007
-
- Towheed TE, Anastassiades T. Glucosamine therapy for osteoarthritis: An update. Journal of Rheumatology 2007;34(9):1787‐90. - PubMed
Vidal 1978
-
- Vidal Y, Plana R, Bizzarri D, Rovati AL. Articular cartilage pharmacology. In vitro studies on glucosamine and non‐steroidal anti‐inflammatory drugs. Pharmacological Research Communications 1978;10:557‐69. - PubMed
Vlad 2007
-
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ?. Arthritis and Rheumatism 2007;56(7):2105‐10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

