The dynamics and mechanics of endothelial cell spreading
- PMID: 15849250
- PMCID: PMC1366566
- DOI: 10.1529/biophysj.104.054320
The dynamics and mechanics of endothelial cell spreading
Abstract
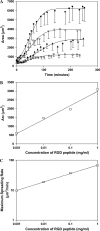
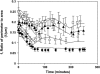
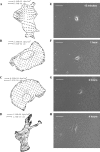
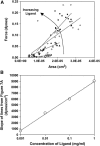
Cell adhesion to extracellular matrix is mediated by receptor-ligand interactions. When a cell first contacts a surface, it spreads, exerting traction forces against the surface and forming new bonds as its contact area expands. Here, we examined the changes in shape, actin polymerization, focal adhesion formation, and traction stress generation that accompany spreading of endothelial cells over a period of several hours. Bovine aortic endothelial cells were plated on polyacrylamide gels derivatized with a peptide containing the integrin binding sequence RGD, and changes in shape and traction force generation were measured. Notably, both the rate and extent of spreading increase with the density of substrate ligand. There are two prominent modes of spreading: at higher surface ligand densities cells tend to spread isotropically, whereas at lower densities of ligand the cells tend to spread anisotropically, by extending pseudopodia randomly distributed along the cell membrane. The extension of pseudopodia is followed by periods of growth in the cell body to interconnect these extensions. These cycles occur at very regular intervals and, furthermore, the extent of pseudopodial extension can be diminished by increasing the ligand density. Measurement of the traction forces exerted by the cell reveals that a cell is capable of exerting significant forces before either notable focal adhesion or stress fiber formation. Moreover, the total magnitude of force exerted by the cell is linearly related to the area of the cell during spreading. This study is the first to monitor the dynamic changes in the cell shape, spreading rate, and forces exerted during the early stages (first several hours) of endothelial cell adhesion.
Figures






References
-
- Asthagiri, A. R., C. A. Reinhart, A. F. Horwitz, and D. A. Lauffenburger. 2000. The role of transient ERK2 signals in fibronectin- and insulin-mediated DNA synthesis. J. Cell Sci. 113:4499–4510. - PubMed
-
- Gospodarowicz, D., and G. M. Lui. 1981. Effect of substrata and fibroblast growth factor on the proliferation in vitro of bovine aortic endothelial cells. J. Cell. Physiol. 109:69–81. - PubMed
-
- McAuslan, B. R., V. Bender, W. Reilly, and B. A. Moss. 1985. New functions of epidermal growth factor: stimulation of capillary endothelial cell migration and matrix dependent proliferation. Cell Biol. Int. Rep. 9:175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

