COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation
- PMID: 15849274
- PMCID: PMC1143074
- DOI: 10.1105/tpc.105.031732
COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation
Abstract
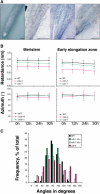
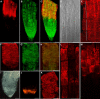
The orientation of cell expansion is a process at the heart of plant morphogenesis. Cellulose microfibrils are the primary anisotropic material in the cell wall and thus are likely to be the main determinant of the orientation of cell expansion. COBRA (COB) has been identified previously as a potential regulator of cellulose biogenesis. In this study, characterization of a null allele, cob-4, establishes the key role of COB in controlling anisotropic expansion in most developing organs. Quantitative polarized-light and field-emission scanning electron microscopy reveal that loss of anisotropic expansion in cob mutants is accompanied by disorganization of the orientation of cellulose microfibrils and subsequent reduction of crystalline cellulose. Analyses of the conditional cob-1 allele suggested that COB is primarily implicated in microfibril deposition during rapid elongation. Immunodetection analysis in elongating root cells revealed that, in agreement with its substitution by a glycosylphosphatidylinositol anchor, COB was polarly targeted to both the plasma membrane and the longitudinal cell walls and was distributed in a banding pattern perpendicular to the longitudinal axis via a microtubule-dependent mechanism. Our observations suggest that COB, through its involvement in cellulose microfibril orientation, is an essential factor in highly anisotropic expansion during plant morphogenesis.
Figures






References
-
- Arioli, T., et al. (1998). Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720. - PubMed
-
- Baskin, T.I. (2001). On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 215, 150–171. - PubMed
-
- Baskin, T.I., Wilson, J.E., Cork, A., and Williamson, R.E. (1994). Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol. 35, 935–942. - PubMed
-
- Benfey, P.N., Linstead, P.J., Roberts, K., Schiefelbein, J.W., Hauser, M.T., and Aeschbacher, R.A. (1993). Root development in Arabidopsis: Four mutants with dramatically altered root morphogenesis. Development 119, 57–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

