Sanguinarine biosynthesis is associated with the endoplasmic reticulum in cultured opium poppy cells after elicitor treatment
- PMID: 15849302
- PMCID: PMC1104173
- DOI: 10.1104/pp.105.059287
Sanguinarine biosynthesis is associated with the endoplasmic reticulum in cultured opium poppy cells after elicitor treatment
Abstract
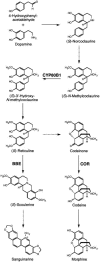
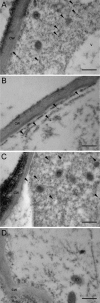
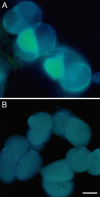
Three key benzylisoquinoline alkaloid biosynthetic enzymes, (S)-N-methylcoclaurine-3'-hydroxylase (CYP80B1), berberine bridge enzyme (BBE), and codeinone reductase (COR), were localized in cultured opium poppy (Papaver somniferum) cells by sucrose density gradient fractionation and immunogold labeling. CYP80B1 catalyzes the second to last step in the formation of (S)-reticuline, the last common intermediate in sanguinarine and morphine biosynthesis. BBE converts (S)-reticuline to (S)-scoulerine as the first committed step in sanguinarine biosynthesis, and COR catalyzes the penultimate step in the branch pathway leading to morphine. Sanguinarine is an antimicrobial alkaloid that accumulates in the vacuoles of cultured opium poppy cells in response to elicitor treatment, whereas the narcotic analgesic morphine, which is abundant in opium poppy plants, is not produced in cultured cells. CYP80B1 and BBE were rapidly induced to high levels in response to elicitor treatment. By contrast, COR levels were constitutive in the cell cultures, but remained low and were not induced by addition of the elicitor. Western blots performed on protein homogenates from elicitor-treated cells fractionated on a sucrose density gradient showed the cosedimentation of CYP80B1, BBE, and sanguinarine with calreticulin, and COR with glutathione S-transferase. Calreticulin and glutathione S-transferase are markers for the endoplasmic reticulum (ER) and the cytosol, respectively. In response to elicitor treatment, large dilated vesicles rapidly developed from the lamellar ER of control cells and fused with the central vacuole. Immunogold localization supported the association of CYP80B1 and BBE with ER vesicles, and COR with the cytosol in elicitor-treated cells. Our results show that benzylisoquinoline biosynthesis and transport to the vacuole are associated with the ER, which undergoes major ultrastructural modification in response to the elicitor treatment of cultured opium poppy cells.
Figures





References
-
- Allen RS, Millgate AG, Chitty JA, Thisleton J, Miller JAC, Fist AJ, Gerlach WL, Larkin PJ (2004) RNAi-mediated replacement of morphine with the non-narcotic alkaloid reticuline in opium poppy. Nat Biotechnol 22: 1559–1566 - PubMed
-
- Amann M, Wanner G, Zenk MH (1986) Intracellular compartmentation of two enzymes of berberine biosynthesis in plant cell cultures. Planta 167: 310–320 - PubMed
-
- Bauer W, Zenk MH (1989) Formation of both methylenedioxy groups in the alkaloid (S)-stylopine is catalyzed by cytochrome P-450 enzymes. Tetrahedron Lett 30: 5257–5260
-
- Bauer W, Zenk MH (1991) Two methylenedioxy bridge forming cytochrome P-450 dependent enzymes are involved in (S)-stylopine biosynthesis. Phytochemistry 30: 2953–2962
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

