Nematode infection triggers the de novo formation of unloading phloem that allows macromolecular trafficking of green fluorescent protein into syncytia
- PMID: 15849304
- PMCID: PMC1104191
- DOI: 10.1104/pp.104.058800
Nematode infection triggers the de novo formation of unloading phloem that allows macromolecular trafficking of green fluorescent protein into syncytia
Abstract
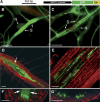
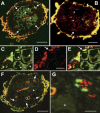
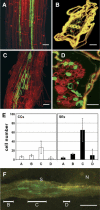
Syncytial feeding complexes induced by the cyst nematode Heterodera schachtii represent strong metabolic sinks for photoassimilates. These newly formed structures were described to be symplastically isolated from the surrounding root tissue and their mechanism of carbohydrate import has repeatedly been under investigation. Here, we present analyses of the symplastic connectivity between the root phloem and these syncytia in nematode-infected Arabidopsis (Arabidopsis thaliana) plants expressing the gene of the green fluorescent protein (GFP) or of different GFP fusions under the control of the companion cell (CC)-specific AtSUC2 promoter. In the same plants, phloem differentiation during syncytium formation was monitored using cell-specific antibodies for CCs or sieve elements (SEs). Our results demonstrate that free, CC-derived GFP moved freely from the phloem into the syncytial domain. No or only marginal cell-to-cell passage of GFP was observed into other root cells adjacent to these syncytia. In contrast, membrane-anchored GFP variants as well as soluble GFP fusions with increased molecular masses were restricted to the SE-CC complex. The presented data also show that nematode infection triggers the de novo formation of phloem containing an approximately 3-fold excess of SEs over CCs. This newly formed phloem exhibits typical properties of unloading phloem previously described in other sink tissues. Our results reveal the existence of a symplastic pathway between phloem CCs and nematode-induced syncytia. The plasmodesmata responsible for this symplastic connectivity allow the cell-to-cell movement of macromolecules up to 30 kD and are likely to represent the major or exclusive path for the supply of assimilates from the phloem into the syncytial complex.
Figures

Similar articles
-
Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots.Plant J. 2005 Jan;41(2):319-31. doi: 10.1111/j.1365-313X.2004.02298.x. Plant J. 2005. PMID: 15634207
-
Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues.Plant Cell. 1999 Mar;11(3):309-22. doi: 10.1105/tpc.11.3.309. Plant Cell. 1999. PMID: 10072393 Free PMC article.
-
Females and males of root-parasitic cyst nematodes induce different symplasmic connections between their syncytial feeding cells and the phloem in Arabidopsis thaliana.Plant Physiol Biochem. 2006 May-Jun;44(5-6):430-3. doi: 10.1016/j.plaphy.2006.06.006. Epub 2006 Jun 15. Plant Physiol Biochem. 2006. PMID: 16889976
-
Plant-nematode interactions.Plant Mol Biol. 1993 Dec;23(5):917-31. doi: 10.1007/BF00021809. Plant Mol Biol. 1993. PMID: 8260631 Review. No abstract available.
-
Control of phloem unloading and root development.J Plant Physiol. 2024 Apr;295:154203. doi: 10.1016/j.jplph.2024.154203. Epub 2024 Feb 17. J Plant Physiol. 2024. PMID: 38428153 Review.
Cited by
-
The other side of the coin: systemic effects of Serendipita indica root colonization on development of sedentary plant-parasitic nematodes in Arabidopsis thaliana.Planta. 2024 Apr 14;259(5):121. doi: 10.1007/s00425-024-04402-5. Planta. 2024. PMID: 38615288 Free PMC article.
-
Starch as a sugar reservoir for nematode-induced syncytia.Plant Signal Behav. 2008 Nov;3(11):961-2. doi: 10.4161/psb.6075. Plant Signal Behav. 2008. PMID: 19704419 Free PMC article.
-
Diversity and activity of sugar transporters in nematode-induced root syncytia.J Exp Bot. 2009;60(11):3085-95. doi: 10.1093/jxb/erp138. Epub 2009 Jun 1. J Exp Bot. 2009. PMID: 19487386 Free PMC article.
-
The plant cell wall in the feeding sites of cyst nematodes.Front Plant Sci. 2014 Mar 19;5:89. doi: 10.3389/fpls.2014.00089. eCollection 2014. Front Plant Sci. 2014. PMID: 24678316 Free PMC article. Review.
-
Altered sucrose synthase and invertase expression affects the local and systemic sugar metabolism of nematode-infected Arabidopsis thaliana plants.J Exp Bot. 2014 Jan;65(1):201-12. doi: 10.1093/jxb/ert359. Epub 2013 Nov 1. J Exp Bot. 2014. PMID: 24187419 Free PMC article.
References
-
- Böckenhoff A, Grundler FMW (1994) Studies on the nutrient uptake by the beet cyst nematode H. schachtii by in situ microinjection of fluorescent probes into the feeding structures in Arabidopsis thaliana. Parasitology 109: 249–254
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

