Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation
- PMID: 15849310
- PMCID: PMC1104170
- DOI: 10.1104/pp.104.055483
Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation
Abstract
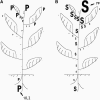
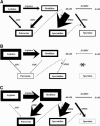
Polyamine (PA) titers and biosynthesis follow a basipetal decrease along the tobacco (Nicotiana tabacum) plant axis, and they also correlate negatively with cell size. On the contrary, the titers of arginine (Arg), ornithine (Orn), and arginase activity increase with age. The free (soluble)/total-PA ratios gradually increase basipetally, but the soluble conjugated decrease, with spermidine (Spd) mainly to determine these changes. The shoot apical meristems are the main site of Spd and spermine biosynthesis, and the hypogeous tissues synthesize mostly putrescine (Put). High and low Spd syntheses are correlated with cell division and expansion, respectively. Put biosynthetic pathways are differently regulated in hyper- and hypogeous tobacco tissues: Only Arg decarboxylase is responsible for Put synthesis in old hypergeous vascular tissues, whereas, in hypogeous tissues, arginase-catalyzed Orn produces Put via Orn decarboxylase. Furthermore, Orn decarboxylase expression coincides with early cell divisions in marginal sectors of the lamina, and Spd synthase strongly correlates with later cell divisions in the vascular regions. This detailed spatial and temporal profile of the free, soluble-conjugated, and insoluble-conjugated fractions of Put, Spd, and spermine in nearly all tobacco plant organs and the profile of enzymes of PA biosynthesis at the transcript, protein, and specific activity levels, along with the endogenous concentrations of the precursor amino acids Arg and Orn, offer new insight for further understanding the physiological role(s) of PAs. The results are discussed in the light of age dependence, cell division/expansion, differentiation, phytohormone gradients, senescence, and sink-source relationships.
Figures




References
-
- Alabadí D, Carbonell J (1999) Molecular cloning and characterization of a tomato (Lycopersicon esculentum Mill.) spermidine synthase cDNA (accession no. AJ006414). (PGR 99–103). Plant Physiol 120: 935
-
- Aziz A (2003) Spermidine and related-metabolic inhibitors modulate sugar and amino acid levels in Vitis vinifera L.: possible relationships with initial fruitlet abscission. J Exp Bot 54: 355–363 - PubMed
-
- Bassie L, Noury M, Lepri O, Lahaye T, Christou P, Capell T (2000) Promoter strength influences polyamine metabolism and morphogenic capacity in transgenic rice tissues expressing the oat adc cDNA constitutively. Transgenic Res 9: 33–42 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

