Influence of RNA structural stability on the RNA chaperone activity of the Escherichia coli protein StpA
- PMID: 15849314
- PMCID: PMC1084320
- DOI: 10.1093/nar/gki515
Influence of RNA structural stability on the RNA chaperone activity of the Escherichia coli protein StpA
Abstract
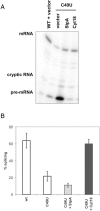
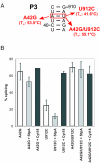
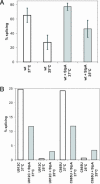
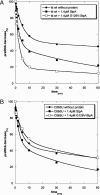
Proteins with RNA chaperone activity are able to promote folding of RNA molecules by loosening their structure. This RNA unfolding activity is beneficial when resolving misfolded RNA conformations, but could be detrimental to RNAs with low thermodynamic stability. In order to test this idea, we constructed various RNAs with different structural stabilities derived from the thymidylate synthase (td) group I intron and measured the effect of StpA, an Escherichia coli protein with RNA chaperone activity, on their splicing activity in vivo and in vitro. While StpA promotes splicing of the wild-type td intron and of mutants with wild-type-like stability, splicing of mutants with a lower structural stability is reduced in the presence of StpA. In contrast, splicing of an intron mutant, which is not destabilized but which displays a reduced population of correctly folded RNAs, is promoted by StpA. The sensitivity of an RNA towards StpA correlates with its structural stability. By lowering the temperature to 25 degrees C, a temperature at which the structure of these mutants becomes more stable, StpA is again able to stimulate splicing. These observations clearly suggest that the structural stability of an RNA determines whether the RNA chaperone activity of StpA is beneficial to folding.
Figures


Similar articles
-
RNA chaperone StpA loosens interactions of the tertiary structure in the td group I intron in vivo.Genes Dev. 2002 Sep 1;16(17):2300-12. doi: 10.1101/gad.231302. Genes Dev. 2002. PMID: 12208852 Free PMC article.
-
RNA chaperone activity and RNA-binding properties of the E. coli protein StpA.Nucleic Acids Res. 2007;35(4):1257-69. doi: 10.1093/nar/gkl1143. Epub 2007 Jan 31. Nucleic Acids Res. 2007. PMID: 17267410 Free PMC article.
-
Assaying RNA chaperone activity in vivo using a novel RNA folding trap.EMBO J. 1999 Jul 1;18(13):3776-82. doi: 10.1093/emboj/18.13.3776. EMBO J. 1999. PMID: 10393192 Free PMC article.
-
Folding of the td pre-RNA with the help of the RNA chaperone StpA.Biochem Soc Trans. 2002 Nov;30(Pt 6):1175-80. doi: 10.1042/bst0301175. Biochem Soc Trans. 2002. PMID: 12440999 Review.
-
Assays for the RNA chaperone activity of proteins.Biochem Soc Trans. 2005 Jun;33(Pt 3):450-6. doi: 10.1042/BST0330450. Biochem Soc Trans. 2005. PMID: 15916539 Review.
Cited by
-
Proteins That Chaperone RNA Regulation.Microbiol Spectr. 2018 Jul;6(4):10.1128/microbiolspec.rwr-0026-2018. doi: 10.1128/microbiolspec.RWR-0026-2018. Microbiol Spectr. 2018. PMID: 30051798 Free PMC article. Review.
-
Kinetic redistribution of native and misfolded RNAs by a DEAD-box chaperone.Nature. 2007 Oct 25;449(7165):1014-8. doi: 10.1038/nature06235. Nature. 2007. PMID: 17960235 Free PMC article.
-
Self-splicing of a group I intron reveals partitioning of native and misfolded RNA populations in yeast.RNA. 2006 Dec;12(12):2149-59. doi: 10.1261/rna.184206. Epub 2006 Oct 24. RNA. 2006. PMID: 17135489 Free PMC article.
-
Taming free energy landscapes with RNA chaperones.RNA Biol. 2010 Nov-Dec;7(6):677-86. doi: 10.4161/rna.7.6.13615. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21045544 Free PMC article. Review.
-
DEAD-box protein CYT-19 is activated by exposed helices in a group I intron RNA.Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):E2928-36. doi: 10.1073/pnas.1404307111. Epub 2014 Jul 7. Proc Natl Acad Sci U S A. 2014. PMID: 25002474 Free PMC article.
References
-
- Treiber D.K., Williamson J.R. Beyond kinetic traps in RNA folding. Curr. Opin. Struct. Biol. 2001;11:309–314. - PubMed
-
- Herschlag D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 1995;270:20871–20874. - PubMed
-
- Schroeder R., Barta A., Semrad K. Strategies for RNA folding and assembly. Nat. Rev. Mol. Cell. Biol. 2004;5:908–919. - PubMed
-
- Karpel R.L., Miller N.S., Fresco J.R. Mechanistic studies of ribonucleic acid renaturation by a helix-destabilizing protein. Biochemistry. 1982;21:2102–2108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

