Diaphragm dysfunction in chronic obstructive pulmonary disease
- PMID: 15849324
- PMCID: PMC2718467
- DOI: 10.1164/rccm.200502-262OC
Diaphragm dysfunction in chronic obstructive pulmonary disease
Abstract
Rationale: Hypercapnic respiratory failure because of inspiratory muscle weakness is the most important cause of death in chronic obstructive pulmonary disease (COPD). However, the pathophysiology of failure of the diaphragm to generate force in COPD is in part unclear.
Objectives: The present study investigated contractile function and myosin heavy chain content of diaphragm muscle single fibers from patients with COPD.
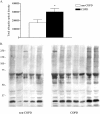
Methods: Skinned muscle fibers were isolated from muscle biopsies from the diaphragm of eight patients with mild to moderate COPD and five patients without COPD (mean FEV(1) % predicted, 70 and 100%, respectively). Contractile function of single fibers was assessed, and afterwards, myosin heavy chain content was determined in these fibers. In diaphragm muscle homogenates, the level of ubiquitin-protein conjugation was determined.
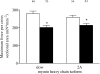
Results: Diaphragm muscle fibers from patients with COPD showed reduced force generation per cross-sectional area, and reduced myosin heavy chain content per half sarcomere. In addition, these fibers had decreased Ca2+ sensitivity of force generation, and slower cross-bridge cycling kinetics. Our observations were present in fibers expressing slow and 2A isoforms of myosin heavy chain. Ubiquitin-protein conjugation was increased in diaphragm muscle homogenates of patients with mild to moderate COPD.
Conclusions: Early in the development of COPD, diaphragm fiber contractile function is impaired. Our data suggest that enhanced diaphragm protein degradation through the ubiquitin-proteasome pathway plays a role in loss of contractile protein and, consequently, failure of the diaphragm to generate force.
Figures





References
-
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med 1998;4:1241–1243. - PubMed
-
- Begin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:905–912. - PubMed
-
- Zielinski J, MacNee W, Wedzicha J, Ambrosino N, Braghiroli A, Dolensky J, Howard P, Gorzelak K, Lahdensuo A, Strom K, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997;52:43–47. - PubMed
-
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:961–966. - PubMed
-
- Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, Marrash R, Pariente R, Aubier M. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol 1998;274:L527–L534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

