Targeted and restricted complement activation on acrosome-reacted spermatozoa
- PMID: 15849610
- PMCID: PMC1077172
- DOI: 10.1172/JCI23213
Targeted and restricted complement activation on acrosome-reacted spermatozoa
Abstract
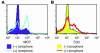
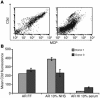
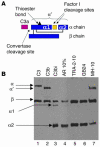
A specific hypoglycosylated isoform of the complement regulator membrane cofactor protein (MCP; CD46) is expressed on the inner acrosomal membrane (IAM) of spermatozoa. This membrane is exposed after the acrosome reaction, an exocytosis event that occurs upon contact with the zona pellucida. We initiated this investigation to assess MCP's regulatory function in situ on spermatozoa. Upon exposure of human spermatozoa to autologous serum or follicular fluid, we unexpectedly observed that acrosome-reacted spermatozoa activated the complement cascade efficiently through C3 but not beyond. Using FACS to simultaneously evaluate viability, acrosomal status, and complement deposition, we found that complement activation was initiated by C-reactive protein (CRP) and was C1q, C2, and factor B dependent. This pattern is consistent with engagement of the classical pathway followed by amplification through the alternative pathway. C3b deposition was targeted to the IAM, where it was cleaved to C3bi. Factor H, and not MCP, was the cofactor responsible for C3b cleavage. We propose that this localized deposition of complement fragments aids in the fusion process between the spermatozoa and egg, in a role akin to that of complement in immune adherence. In addition, we speculate that this "targeted and restricted" form of complement activation on host cells is a common strategy to handle modified self.
Figures

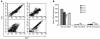


References
-
- Ballard LL, Bora NS, Yu GH, Atkinson JP. Biochemical characterization of membrane cofactor protein of the complement system. J. Immunol. 1988;141:3923–3929. - PubMed
-
- Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

