Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver
- PMID: 15849611
- PMCID: PMC1077176
- DOI: 10.1172/JCI24356
Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver
Abstract
Solute carrier family 11, member 2 (SLC11A2) is the only transmembrane iron transporter known to be involved in cellular iron uptake. It is widely expressed and has been postulated to play important roles in intestinal iron absorption, erythroid iron utilization, hepatic iron accumulation, placental iron transfer, and other processes. Previous studies have suggested that other transporters might exist, but their physiological significance remained uncertain. To define the activities of Slc11a2 in vivo, we inactivated the murine gene that encodes it globally and selectively. We found that fetal Slc11a2 is not needed for materno-fetal iron transfer but that Slc11a2 activity is essential for intestinal non-heme iron absorption after birth. Slc11a2 is also required for normal hemoglobin production during the development of erythroid precursors. However, hepatocytes and most other cells must have an alternative, as-yet-unknown, iron uptake mechanism. We previously showed that Slc11a2 serves as the primary portal for intestinal iron entry in hemochromatosis. However, inactivation of murine Hfe ameliorates the phenotype of animals lacking Slc11a2.
Figures


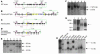
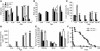
References
-
- Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 1999;21:396–399. - PubMed
-
- Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. - PubMed
-
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. - PubMed
-
- Canonne-Hergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood. 2001;98:3823–3830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

