In vitro assembly and GTP hydrolysis by bacterial tubulins BtubA and BtubB
- PMID: 15851515
- PMCID: PMC2171859
- DOI: 10.1083/jcb.200410027
In vitro assembly and GTP hydrolysis by bacterial tubulins BtubA and BtubB
Abstract
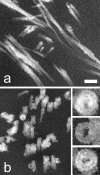
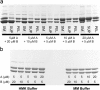
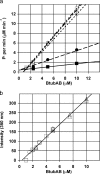
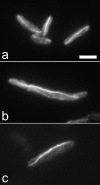
Arecent study identified genuine tubulin proteins, BtubA and BtubB, in the bacterial genus Prosthecobacter. We have expressed BtubA and BtubB in Escherichia coli and studied their in vitro assembly. BtubB by itself formed rings with an outer diameter of 35-36 nm in the presence of GTP or GDP. Mixtures of BtubB and BtubA formed long protofilament bundles, 4-7 protofilaments wide (20-30 protofilaments in the three-dimensional bundle). Regardless of the starting stoichiometry, the polymers always contained equal concentrations of BtubA and BtubB, suggesting that BtubA and B alternate along the protofilament. BtubA showed negligible GTP hydrolysis, whereas BtubB hydrolyzed 0.40 mol GTP per min per mol BtubB. This GTPase activity increased to 1.37 per min when mixed 1:1 with BtubA. A critical concentration of 0.4-1.0 microM was indicated by light scattering experiments and extrapolation of GTPase versus concentration, thus suggesting a cooperative assembly mechanism.
Figures

References
-
- Bock, R.M., N.S. Ling, S.A. Morell, and S.H. Lipton. 1956. Ultraviolet absorption spectra of adenosine-5′-triphosphate and related 5′-ribonucleotides. Arch. Biochem. Biophys. 62:253–264. - PubMed
-
- Cayley, S., B.A. Lewis, H.J. Guttman, and M.T. Record Jr. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity: implications for protein-DNA interactions in vivo. J. Mol. Biol. 222:281–300. - PubMed

