Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes
- PMID: 15851656
- PMCID: PMC1088394
- DOI: 10.1073/pnas.0502467102
Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes
Abstract
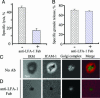
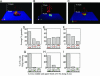
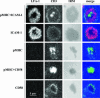
Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecules (ICAMs) facilitates T cell antigen receptor (TCR)-mediated killing. To dissect TCR and LFA-1 contributions, we evaluated cytolytic activity and granule release by cytotoxic T lymphocytes (CTL) as well as intracellular granule redistribution and morphology of CTL stimulated with natural TCR ligand in the presence or absence of LFA-1 engagement. Although other adhesion mechanisms, e.g., CD2-CD58 interaction, could substitute for LFA-1 to trigger CTL degranulation, productive LFA-1 ligation was indispensable for effective target cell lysis by the released granules. LFA-1-mediated adhesion to glass-supported bilayers containing intercellular adhesion molecule-1 was characterized by a much larger junction area, marked by LFA-1 segregation, and a more compact cell shape compared with those observed for CD2-mediated adhesion to bilayers containing CD58. A larger contact induced by intercellular adhesion molecule 1 determined a unique positioning of granules near the interface. These data provide evidence that LFA-1 delivers a distinct signal essential for directing released cytolytic granules to the surface of antigen-bearing target cells to mediate the effective destruction of these cells by CTL.
Figures

References
-
- Lowin, B., Hahne, M., Mattmann, C. & Tschopp, J. (1994) Nature 370, 650-652. - PubMed
-
- Berke, G. (1994) Annu. Rev. Immunol. 12, 735-773. - PubMed
-
- Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. (1996) Immunity 4, 565-571. - PubMed
-
- Shaw, S., Luce, G. E., Quinones, R., Gress, R. E., Springer, T. A. & Sanders, M. E. (1986) Nature 323, 262-264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

