Phase 2 ventricular arrhythmias in acute myocardial infarction: a neglected target for therapeutic antiarrhythmic drug development and for safety pharmacology evaluation
- PMID: 15852034
- PMCID: PMC1576179
- DOI: 10.1038/sj.bjp.0706231
Phase 2 ventricular arrhythmias in acute myocardial infarction: a neglected target for therapeutic antiarrhythmic drug development and for safety pharmacology evaluation
Abstract
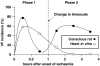
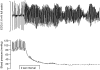
Ventricular fibrillation (VF), a cause of sudden cardiac death (SCD) in the setting of acute myocardial infarction (MI), remains a major therapeutic challenge. In humans, VF may occur within minutes or hours after the onset of chest pain, so its precise timing in relation to the onset of ischaemia is variable. Moreover, because VF usually occurs unobserved, out of hospital, and is usually lethal in the absence of intervention, its precise timing of onset is actually unknown in most patients. In animal models, the timing of susceptibility to VF is much better characterised. It occurs in two distinct phases. Early VF (defined as phase 1 VF, with possible subphases 1a and 1b in some animal species) occurs during the first 30 min of ischaemia when most myocardial injury is still reversible. Late VF, defined as phase 2 VF, occurs when myocardial necrosis is becoming established (after more than 90 min of ischaemia). Although much is known about the mechanisms and pharmacology of phase 1 VF, little is known about phase 2 VF. By reviewing a range of different types of data we have outlined the likely mechanisms and clinical relevance of phase 2 VF, and have evaluated possible future directions to help evolve a strategy for its suppression by drugs. The possibility that a proarrhythmic effect on phase 2 VF contributes to the adverse cardiac effects of certain cardiac and noncardiac drugs is also discussed in relation to the emerging field of safety pharmacology. It is concluded that suppression of phase 2 as well as phase 1 VF will almost certainly be necessary if drugs of the future are to achieve what drugs of the past and present have failed to achieve: full protection against SCD. Likewise, safety will require avoidance of exacerbation of phase 2 as well as phase 1 VF.
Figures
References
-
- AIDONIDIS I., BRACHMANN J., RIZOS I., ZACHAROULIS A., STAVRIDIS I., TOUTOUZAS P., KUBLER W. Electropharmacology of the bradycardic agents alinidine and zatebradine (UL-FS 49) in a conscious canine ventricular arrhythmia model of permanent coronary artery occlusion. Cardiovasc. Drugs Ther. 1995;9:555–563. - PubMed
-
- ALLESSIE A., AVKIRAN M., BORGGREFE M., BRACHMANN J., BREITHARDT G., CAMM A.J., CARMELIET E., CINCA J., COBBE S.M., CURTIS M.J., DHEIN S., HAVERKAMP W., HINDRICKS G., JANSE M.J., KLEBER A.G., KOTTKAMP H., OPHTHOF T., PRIORI S.G., SACK S., SCHOLS W.J., SCHWARTZ P.J., VANOLI E., ZAA A. The role of basic arrhythmia research. The continued need for experiments in the intact heart and organism. Eur. Heart J. 1995;16:1469–1475. - PubMed
-
- ANNABLE C.R., MCMANUS L.M., CAREY K.D., PINCKARD R.N. Isolation of platelet activating factor (PAF) from ischaemic baboon myocardium. Fed. Proc. 1985;44:1271.
-
- ANTMAN E.M., LAU J., KUPELNICK B., MOSTELLER F., CHALMERS T.C. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268:240–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

