The involvement of nitric oxide in the enhanced expression of mu-opioid receptors during intestinal inflammation in mice
- PMID: 15852037
- PMCID: PMC1576189
- DOI: 10.1038/sj.bjp.0706227
The involvement of nitric oxide in the enhanced expression of mu-opioid receptors during intestinal inflammation in mice
Abstract
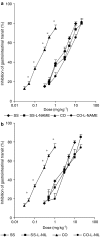
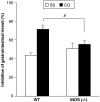
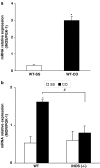
Intestinal inflammation enhances the potency of mu-opioid receptor (MOR) agonists inhibiting gastrointestinal transit and increases the expression of MOR in mice intestine. The precise mechanisms implicated in the increased expression of MOR during intestinal inflammation are not known. The aim of the study is to evaluate if nitric oxide released during intestinal inflammation could modulate MOR gene expression and affect gastrointestinal transit. Intestinal inflammation was induced by the intragastric administration of croton oil. In CD-1 mice, with and without inflammation, we evaluated the anti-transit effects of morphine in animals treated with NOS inhibitors (L-NAME and L-NIL) and the intestinal levels of iNOS enzyme mRNA. The anti-transit effects of morphine and the expression of MOR mRNA in the gut of wild-type (WT) and iNOS-/- mice were also assessed. Gastrointestinal transit was measured with charcoal meal and mRNA levels determined by real-time PCR. In CD-1 mice, inflammation induced a 10-fold increase (P<0.0001) in iNOS mRNA levels in the gut. The absence of iNOS gene and treatment of CD-1 mice with L-NAME or L-NIL abolished the increased antitransit effects of morphine observed during inflammation. Moreover, although the basal levels of MOR mRNA were similar in WT and iNOS animals (-/-), intestinal inflammation only increased the MOR expression in the gut of WT (P<0.01) but not in iNOS-/- mice. The results suggest that nitric oxide derived from the increased expression of iNOS is implicated in the enhanced effects of morphine and in the upregulation of MOR gene transcription observed during intestinal inflammation.
Figures

Similar articles
-
Reversal of tolerance to the antitransit effects of morphine during acute intestinal inflammation in mice.Br J Pharmacol. 1997 Nov;122(6):1216-22. doi: 10.1038/sj.bjp.0701472. Br J Pharmacol. 1997. PMID: 9401789 Free PMC article.
-
Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide.J Leukoc Biol. 2001 Oct;70(4):527-36. J Leukoc Biol. 2001. PMID: 11590188
-
Antisense oligodeoxynucleotides to mu- and delta-opioid receptor mRNA block the enhanced effects of opioids during intestinal inflammation.Eur J Pharmacol. 2001 Sep 28;428(1):127-36. doi: 10.1016/s0014-2999(01)01281-x. Eur J Pharmacol. 2001. PMID: 11779029
-
The involvement of the nitric oxide in the effects and expression of opioid receptors during peripheral inflammation.Curr Med Chem. 2007;14(18):1945-55. doi: 10.2174/092986707781368469. Curr Med Chem. 2007. PMID: 17691937 Review.
-
Inducible nitric oxide synthase: a little bit of good in all of us.Gut. 2000 Jul;47(1):6-9. doi: 10.1136/gut.47.1.6. Gut. 2000. PMID: 10861252 Free PMC article. Review.
Cited by
-
Peripheral effects of morphine and expression of μ-opioid receptors in the dorsal root ganglia during neuropathic pain: nitric oxide signaling.Mol Pain. 2011 Apr 12;7:25. doi: 10.1186/1744-8069-7-25. Mol Pain. 2011. PMID: 21486477 Free PMC article.
-
Milk Intolerance, Beta-Casein and Lactose.Nutrients. 2015 Aug 31;7(9):7285-97. doi: 10.3390/nu7095339. Nutrients. 2015. PMID: 26404362 Free PMC article. Review.
-
Alteration of neuromuscular transmissions in the hamster colon following the resolution of TNBS-induced colitis.J Physiol Sci. 2013 Jul;63(4):241-9. doi: 10.1007/s12576-013-0256-9. Epub 2013 Apr 9. J Physiol Sci. 2013. PMID: 23568479 Free PMC article.
-
Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein.Adv Nutr. 2017 Sep 15;8(5):739-748. doi: 10.3945/an.116.013953. Print 2017 Sep. Adv Nutr. 2017. PMID: 28916574 Free PMC article.
-
Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome.Sci Rep. 2019 Dec 20;9(1):19603. doi: 10.1038/s41598-019-56132-4. Sci Rep. 2019. PMID: 31862976 Free PMC article.
References
-
- BAGNOL D., MANSOUR A., AKIL H., WATSON S.J. Cellular localization and distribution of the cloned μ and κ opioid receptors in rat gastrointestinal tract. Neuroscience. 1997;81:579–591. - PubMed
-
- BECK P.L., XAVIER R., WONG J., EZEDI I., MASHIMO H., MIZOGUCHI A., MIZOGUCHI E., BHAN A.K., PODOLSKY D.K. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G137–G147. - PubMed
-
- BIANCHI M., MAGGI R., PIMPINELLI F., RUBINO T., PAROLARO D., POLI V., CILIBERTO G., PANERAI A.E., SACERDOTE P. Presence of a reduced opioid response in interleukin-6 knockout mice. Eur. J. Neurosci. 1999;11:1501–1507. - PubMed
-
- BÖRNER C., KRAUS J., SCHRÖDER H., AMMER H., HÖLLT V. Transcriptional regulation of the human μ-opioid receptor gene by interleukin-6. Mol. Pharmacol. 2004;66:1719–1726. - PubMed
-
- CHEN H.C., LOH H.H. μ-Opioid receptor gene expression: the role of NCAM. Neuroscience. 2001;108:7–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

