Protecting the blood supply from emerging pathogens: the role of pathogen inactivation
- PMID: 15852240
- PMCID: PMC7126528
- DOI: 10.1016/j.tmrv.2004.11.005
Protecting the blood supply from emerging pathogens: the role of pathogen inactivation
Abstract
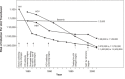
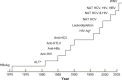
Although the risk of infection by blood transfusion is relatively low, breakthrough infections still occur, Transfusion-related fatalities caused by infections continue to be reported, and blood is not tested for many potentially dangerous pathogens. The current paradigm for increasing the safety of the blood supply is the development and implementation of laboratory screening methods and restrictive donor criteria. When considering the large number of known pathogens and the fact that pathogens continue to emerge, it is clear that the utility of new tests and donor restrictions will continue to be a challenge when considering the cost of developing and implementing new screening assays, the loss of potential donors, and the risk of testing errors. Despite improving the safety of blood components, testing remains a reactive approach to blood safety. The contaminating organisms must be identified before sensitive tests can be developed. In contrast, pathogen inactivation is a proactive strategy designed to inactivate a pathogen before it enters the blood supply. Almost all pathogen inactivation technologies target nucleic acids, allowing for the inactivation of a variety of nucleic acid-containing pathogens within plasma, platelets, or red blood cells thus providing the potential to reduce transfusion-transmitted diseases. However, widespread use of a pathogen inactivation technology can only be realized when proven safe and efficacious and not cost-prohibitive.
Figures

Similar articles
-
Pathogen inactivation: a new paradigm for preventing transfusion-transmitted infections.Am J Clin Pathol. 2007 Dec;128(6):945-55. doi: 10.1309/RAPQ3NXG3MV9AL94. Am J Clin Pathol. 2007. PMID: 18024320 Free PMC article. Review.
-
Update of pathogen reduction technology for therapeutic plasma.Mymensingh Med J. 2010 Apr;19(2):308-11. Mymensingh Med J. 2010. PMID: 20395932 Review.
-
Pathogen-reduction systems for blood components: the current position and future trends.Transfus Apher Sci. 2006 Dec;35(3):189-96. doi: 10.1016/j.transci.2006.10.002. Epub 2006 Nov 15. Transfus Apher Sci. 2006. PMID: 17110168 Review.
-
Inactivation of infectious pathogens in labile blood components: meeting the challenge.Transfus Clin Biol. 2001 Jun;8(3):138-45. doi: 10.1016/s1246-7820(01)00117-3. Transfus Clin Biol. 2001. PMID: 11499954 Review.
-
Pathogen inactivation: a new paradigm for blood safety.Transfusion. 2007 Dec;47(12):2180-4. doi: 10.1111/j.1537-2995.2007.01539.x. Transfusion. 2007. PMID: 18036073 No abstract available.
Cited by
-
Inactivation of human plasma alters the structure and biomechanical properties of engineered tissues.Front Bioeng Biotechnol. 2022 Aug 23;10:908250. doi: 10.3389/fbioe.2022.908250. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36082161 Free PMC article.
-
Listeria monocytogenes Transmission from Donated Blood to Platelet Transfusion Recipient, Italy.Emerg Infect Dis. 2023 Oct;29(10):2108-21011. doi: 10.3201/eid2910.230746. Epub 2023 Jul 21. Emerg Infect Dis. 2023. PMID: 37478295 Free PMC article.
-
The influence of riboflavin photochemistry on plasma coagulation factors.Transfus Apher Sci. 2009 Dec;41(3):199-204. doi: 10.1016/j.transci.2009.09.006. Epub 2009 Sep 25. Transfus Apher Sci. 2009. PMID: 19782644 Free PMC article.
-
Treatment of nonhealing diabetic lower extremity ulcers with skin graft and autologous platelet gel: a case series.Biomed Res Int. 2013;2013:837620. doi: 10.1155/2013/837620. Epub 2013 Mar 31. Biomed Res Int. 2013. PMID: 23607097 Free PMC article.
-
Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality.Transfus Med Rev. 2010 Apr;24(2):77-124. doi: 10.1016/j.tmrv.2009.11.001. Transfus Med Rev. 2010. PMID: 20303034 Free PMC article. Review.
References
-
- Ling A.E., Robbins K.E., Brown T.M. Failure of routine HIV-1 tests in a case involving transmission with preseroconversion blood components during the infectious window period. JAMA. 2000;284:210–214. - PubMed
-
- Glynn S.A., Kleinman S.H., Schreiber G.B. Trends in incidence and prevalence of major transfusion-transmissible viral infections in US blood donors, 1991 to 1996. Retrovirus Epidemiology Donor Study (REDS) JAMA. 2000;284:229–235. - PubMed
-
- American Association of Blood Banks Facts about blood and blood banking. AABB. http://www.aabb.org/All_About_Blood/FAQs/aabb_faqs.htm Available at: [Accessed October 17, 2003]
-
- Busch M.P., Kleinman S.H., Nemo G.J. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–962. - PubMed
-
- Kleinman S.H., Busch M.P. HBV: Amplified and back in the blood safety spotlight. Transfusion. 2001;41:1081–1085. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

