Three-component coupling reactions of silylglyoxylates, alkynes, and aldehydes: a chemoselective one-step glycolate aldol construction
- PMID: 15853312
- PMCID: PMC2827871
- DOI: 10.1021/ja043884l
Three-component coupling reactions of silylglyoxylates, alkynes, and aldehydes: a chemoselective one-step glycolate aldol construction
Abstract
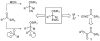
A single-pot three-component coupling reaction of silylglyoxylates (1), terminal alkynes, and aldehydes in the presence of ZnI2 and Et3N is presented. The products of the reaction, densely functionalized silyl-protected glycolate aldols (2), can be converted to the corresponding acetonides (3) in a one-pot deprotection/ketalization sequence. A variety of terminal alkynes and aldehydes can be successfully employed to give a range of highly functionalized, fully protected 1,2-diols in good yields and moderate diastereoselectivities. Mechanistic experiments suggest that the zinc acetylide reacts with the silylgyloxylate (1) in a chemoselective manner. Using an unoptimized (+)-N-methylephedrine and Zn(OTf)2 system, silyl-deprotected adduct 2 was formed in 64% ee and 89:11 dr.
References
-
- Moser WH. Tetrahedron. 2001;57:2065.
-
- Brook AG. Acc Chem Res. 1974;7:77.
-
- Reich HJ, Holtan RC, Bolm C. J Am Chem Soc. 1990;112:5609.
- Takeda K, Ohnishi Y. Tetrahedron Lett. 2000;41:4169.
- Degl’Innocenti A, Ricci A, Mordini A, Reginato G, Colotta V. Gazz Chim Ital. 1987;117:645.
-
- Takeda K, Tanaka T. Synlett. 1999:705.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources