In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets
- PMID: 15855275
- PMCID: PMC1895168
- DOI: 10.1182/blood-2005-02-0509
In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets
Abstract
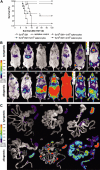
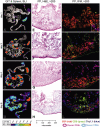
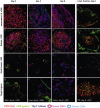
Graft-versus-host disease (GVHD) is a major obstacle in allogeneic hematopoietic cell transplantation. Given the dynamic changes in immune cell subsets and tissue organization, which occur in GVHD, localization and timing of critical immunological events in vivo may reveal basic pathogenic mechanisms. To this end, we transplanted luciferase-labeled allogeneic splenocytes and monitored tissue distribution by in vivo bioluminescence imaging. High-resolution analyses showed initial proliferation of donor CD4+ T cells followed by CD8+ T cells in secondary lymphoid organs with subsequent homing to the intestines, liver, and skin. Transplantation of purified naive T cells caused GVHD that was initiated in secondary lymphoid organs followed by target organ manifestation in gut, liver, and skin. In contrast, transplanted CD4+ effector memory T (T(EM)) cells did not proliferate in secondary lymphoid organs in vivo and despite their in vitro alloreactivity in mixed leukocyte reaction (MLR) assays did not cause acute GVHD. These findings underline the potential of T-cell subsets with defined trafficking patterns for immune reconstitution without the risk of GVHD.
Figures
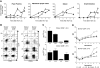

References
-
- Horrowitz M. Uses and growth of hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ, Appelbaum FR, eds. Thomas' Hematopoietic Cell Transplantation. Malden, MA: Blackwell; 2004: 9-15.
-
- Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411: 385-389. - PubMed
-
- Shizuru JA. The experimental basis for hematopoietic cell transplantation for autoimmune diseases. In: Thomas ED, Blume KG, Forman SJ, Appelbaum FR, eds. Thomas' Hematopoietic Cell Transplantation. Malden, MA: Blackwell; 2004: 324-343.
-
- Ferrara JL, Deeg HJ, Division of Pediatric Oncology D-FCIBMA. Graft-versus-host disease. N Engl J Med. 1991;324: 667-674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

