Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses?
- PMID: 15855489
- PMCID: PMC1087660
- DOI: 10.1128/AAC.49.5.1733-1738.2005
Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses?
Abstract
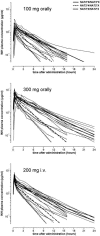
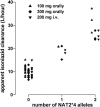
Isoniazid is metabolized by the genetically polymorphic arylamine N-acetyltransferase type 2 (NAT2). A greater number of high-activity alleles are related to increased acetylation capacity and in some reports to low efficacy and toxicity of isoniazid. The objective of this study was to assess individual isoniazid exposure based on NAT2 genotype to predict a personalized therapeutic dose. Isoniazid was administered to 18 healthy Caucasians (age 30 +/- 6 years, body weight 74 +/- 10 kg, five women) in random order as a 200-mg infusion, a 100-mg oral, and a 300-mg oral single dose. For the assessment of NAT2 genotype, common single nucleotide polymorphisms identifying 99.9% of variant alleles were characterized. Noncompartmental pharmacokinetics and compartmental population pharmacokinetics were estimated from isoniazid plasma concentrations until 24 h postdose by high-pressure liquid chromatography. The influence of NAT2 genotype, drug formulation, body weight, and sex on dose-normalized isoniazid pharmacokinetics was assessed by analysis of variance from noncompartmental data and confirmed by population pharmacokinetics. Eight high-activity NAT2*4 alleles were identified. Sex had no effect; the other factors explained 93% of the variability in apparent isoniazid clearance (analysis of variance). NAT2 genotype alone accounted for 88% of variability. Individual isoniazid clearance could be predicted as clearance (liters/hour) = 10 + 9 x (number of NAT2*4 alleles). To achieve similar isoniazid exposure, current standard doses presumably appropriate for patients with one high-activity NAT2 allele may be decreased or increased by approximately 50% for patients with no or two such alleles, respectively. Prospective clinical trials are required to assess the merits of this approach.
Figures

References
-
- Cascorbi, I., and I. Roots. 1999. Pitfalls in N-acetyltransferase 2 genotyping. Pharmacogenetics. 9:123-127. - PubMed
-
- Clark, D. W. 1985. Genetically determined variability in acetylation and oxidation. Therapeutic implications. Drugs 29:342-375. - PubMed
-
- Donald, P. R., F. A. Sirgel, F. J. Botha, H. I. Seifart, D. P. Parkin, M. L. Vandenplas, B. W. Van de Wal, J. S. Maritz, and D. A. Mitchison. 1997. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156:895-900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

