Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein
- PMID: 15855507
- PMCID: PMC1087643
- DOI: 10.1128/AAC.49.5.1857-1864.2005
Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein
Abstract
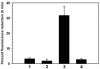
Efflux is an important mechanism of multidrug resistance (MDR) in bacteria. The multidrug and toxin extrusion (MATE) family is the most recently described group of MDR efflux proteins, none of which have previously been identified in Staphylococcus aureus. Two independently derived S. aureus mutants having efflux-related MDR phenotypes were studied using microarray technology and a marked overexpression of an open reading frame (ORF; mepA) encoding a protein homologous with MATE family proteins was observed in both. There was concomitant overexpression of ORFs in close proximity to mepA (approximately 100 bp) encoding a MarR-type regulator (mepR, upstream of mepA) and a protein of unknown function (mepB, downstream). Experiments in which mepA was overexpressed or disrupted revealed that the encoded protein has a broad substrate profile that includes several monovalent and divalent biocides and the fluoroquinolone antimicrobial agents norfloxacin and ciprofloxacin. The function of MepB is obscure, it does not contribute to the MDR phenotype conferred by MepA. MepR overexpression reversed the MDR phenotypes of both mutants by repressing mepA transcription. All three ORFs are preferentially transcribed as a single mepRAB unit, suggesting that the three genes form an operon.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

