Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue
- PMID: 15855512
- PMCID: PMC1087627
- DOI: 10.1128/AAC.49.5.1898-1906.2005
Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue
Abstract
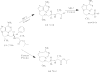
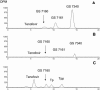
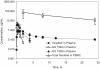
An isopropylalaninyl monoamidate phenyl monoester prodrug of tenofovir (GS 7340) was prepared, and its in vitro antiviral activity, metabolism, and pharmacokinetics in dogs were determined. The 50% effective concentration (EC(50)) of GS 7340 against human immunodeficiency virus type 1 in MT-2 cells was 0.005 microM compared to an EC(50) of 5 microM for the parent drug, tenofovir. The (L)-alaninyl analog (GS 7340) was >1,000-fold more active than the (D)-alaninyl analog. GS 7340 has a half-life of 90 min in human plasma at 37 degrees C and a half-life of 28.3 min in an MT-2 cell extract at 37 degrees C. The antiviral activity (>10 x the EC(50)) and the metabolic stability in MT-2 cell extracts (>35 x) and plasma (>2.5 x) were also sensitive to the stereochemistry at the phosphorus. After a single oral dose of GS 7340 (10 mg-eq/kg tenofovir) to male beagle dogs, the plasma bioavailability of tenofovir compared to an intravenous dose of tenofovir was 17%. The total intracellular concentration of all tenofovir species in isolated peripheral blood mononuclear cells at 24 h was 63 microg-eq/ml compared to 0.2 microg-eq/ml in plasma. A radiolabeled distribution study with dogs resulted in an increased distribution of tenofovir to tissues of lymphatic origin compared to the commercially available prodrug tenofovir DF (Viread).
Figures
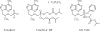


Similar articles
-
Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases.Mol Pharmacol. 2008 Jul;74(1):92-100. doi: 10.1124/mol.108.045526. Epub 2008 Apr 22. Mol Pharmacol. 2008. PMID: 18430788
-
Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131.Antimicrob Agents Chemother. 2008 Feb;52(2):655-65. doi: 10.1128/AAC.01215-07. Epub 2007 Dec 3. Antimicrob Agents Chemother. 2008. PMID: 18056282 Free PMC article.
-
Novel nucleotide human immunodeficiency virus reverse transcriptase inhibitor GS-9148 with a low nephrotoxic potential: characterization of renal transport and accumulation.Antimicrob Agents Chemother. 2009 Jan;53(1):150-6. doi: 10.1128/AAC.01183-08. Epub 2008 Nov 10. Antimicrob Agents Chemother. 2009. PMID: 19001108 Free PMC article.
-
Tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of HIV infection.Clin Ther. 2002 Oct;24(10):1515-48. doi: 10.1016/s0149-2918(02)80058-3. Clin Ther. 2002. PMID: 12462284 Review.
-
[Tenofovir: pharmacology and interactions].Enferm Infecc Microbiol Clin. 2008 Jun;26 Suppl 8:2-6. doi: 10.1157/13126265. Enferm Infecc Microbiol Clin. 2008. PMID: 19195431 Review. Spanish.
Cited by
-
Metabolic Efficacy of Phosphate Prodrugs and the Remdesivir Paradigm.ACS Pharmacol Transl Sci. 2020 Jul 24;3(4):613-626. doi: 10.1021/acsptsci.0c00076. eCollection 2020 Aug 14. ACS Pharmacol Transl Sci. 2020. PMID: 32821882 Free PMC article. Review.
-
Evaluating emtricitabine + rilpivirine + tenofovir alafenamide in combination for the treatment of HIV-infection.Expert Opin Pharmacother. 2020 Mar;21(4):389-397. doi: 10.1080/14656566.2020.1713096. Epub 2020 Jan 20. Expert Opin Pharmacother. 2020. PMID: 31957507 Free PMC article. Review.
-
Prodrug strategies for improved efficacy of nucleoside antiviral inhibitors.Curr Opin HIV AIDS. 2013 Nov;8(6):556-64. doi: 10.1097/COH.0000000000000007. Curr Opin HIV AIDS. 2013. PMID: 24100876 Free PMC article. Review.
-
Development and validation of an LC-MS/MS assay for tenofovir and tenofovir alafenamide in human plasma and cerebrospinal fluid.J Pharm Biomed Anal. 2018 Jul 15;156:163-169. doi: 10.1016/j.jpba.2018.04.035. Epub 2018 Apr 23. J Pharm Biomed Anal. 2018. PMID: 29709783 Free PMC article.
-
Advances in Nucleotide Antiviral Development from Scientific Discovery to Clinical Applications: Tenofovir Disoproxil Fumarate for Hepatitis B.J Clin Transl Hepatol. 2013 Sep;1(1):33-8. doi: 10.14218/JCTH.2013.004XX. Epub 2013 Sep 15. J Clin Transl Hepatol. 2013. PMID: 26357604 Free PMC article. Review.
References
-
- Arimilli, M., C. Kim, and N. Bischofberger. 1997. Synthesis, in vitro biological evaluation and oral bioavailability of 9-[2-(phosphonomethoxy)- propyl]adenine (PMPA) prodrugs. Antivir. Chem. Chemother. 8:557-564.
-
- Balzarini, J., A. Holy, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2, 6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. - PMC - PubMed
-
- Cundy, K. C. 1999. Presented at the 7th European Conference on Clinical Aspects and Treatment of HIV Infection, Lisbon, Portugal, 23 to 27 October 1999.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

